Answered step by step
Verified Expert Solution
Question
1 Approved Answer
second time asking could really use the help (So3 is not .5 , air is not 3.1, and so2 is not .7) if possible please
second time asking could really use the help (So3 is not .5 , air is not 3.1, and so2 is not .7) if possible please include the heat of reaction 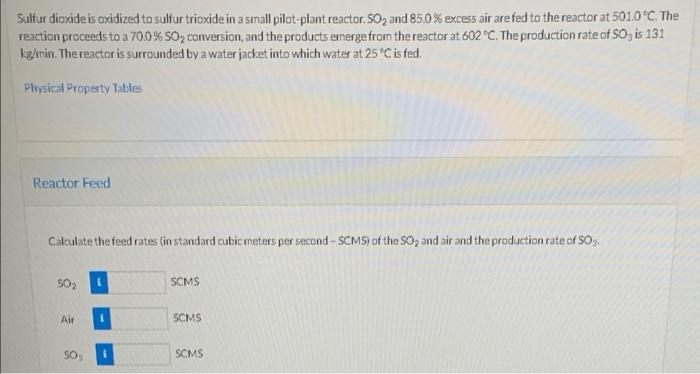

the heat of reaction will be i. kJ
Sulfur dioxide is oxidized to sulfur trioxide in a small pilot-plant reactor. 50, and 85.0% excess air are fed to the reactor at 501.0C. The reaction proceeds to a 70.0% 50, conversion, and the products emerge from the reactor at 602 "C. The production rate of SO, is 131 kg/min. The reactor is surrounded by a water jacket into which water at 25C is fed. Physical Property Tables Reactor Feed Calculate the feed rates (in standard cubic meters per second-SCMS) of the SO2 and air and the production rate of S03. SO, SCMS Air SCMS SO SCMS Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


