Answered step by step
Verified Expert Solution
Question
1 Approved Answer
see image for reference Please explain the answer in brief Find the rate of reaction of A , B , C . - 1 litmir
see image for reference
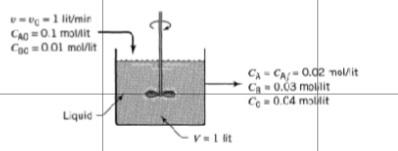
Please explain the answer in brief

Find the rate of reaction of A , B , C .
- 1 litmir 0.1 moMit Coc=001 mol/l CA-C-0.02 nolit G0.03 molit Co0.04 min Liquid V1 lit One liter per minute of liquid containing A and B (CA0 = 0.10 mol/liter, C180 0.01 mol/liter) flow into a mixed reactor of volume V = 1 liter. The materials react in a complex manner for which the stoichiometry is unknown. The outlet stream from the reactor contains A, B, and C (CAY = 0.02 mol/liter, Cny = 0.03 mol/liter, Cy = 0.04 mol/liter), as shown in Fig. E5.1. Find the rate of reaction of A, B, and C for the conditions within the reactorStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


