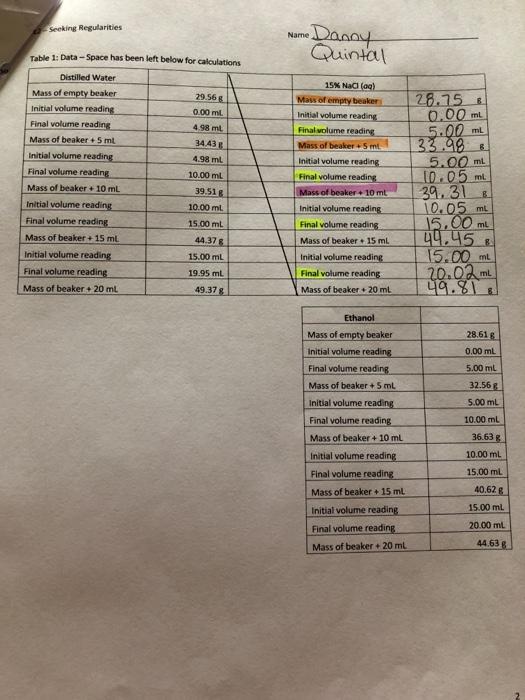
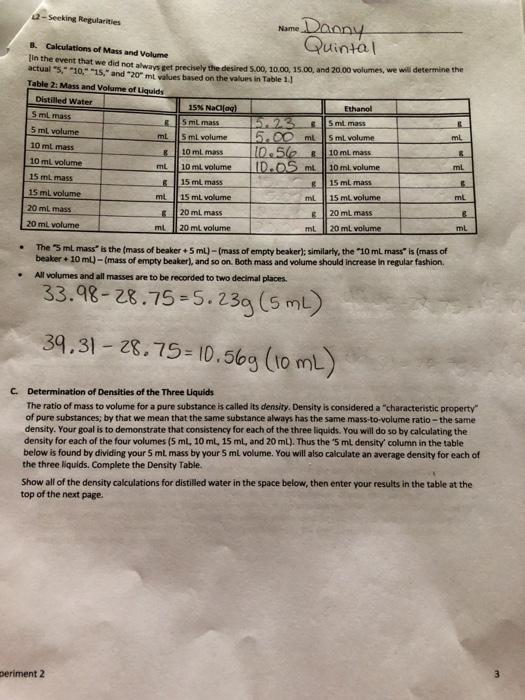
Seeking Regularities Name Danny Quintal 29.56 Table 1: Data-Space has been left below for calculations Distilled Water Mass of empty beaker Initial volume reading 0.00 ml Final volume reading 4 98 ml Mass of beaker + 5 ml 34.43 Initial volume reading 4.98 ml Final volume reading 10.00 ml Mass of beaker + 10 mL 39.518 Initial volume reading 10.00 ml Final volume reading 15.00 ml Mass of beaker + 15 ml 44.37 Initial volume reading 15.00 ml Final volume reading 19.95 ml Mass of beaker + 20 ml 49.378 15 Nadia) Maw of empty beaker Initial volume reading Final volume reading Mass of beaker.5 ml Initial volume reading Final volume reading Mass of beaker + 10 ml Initial volume reading Final volume reading Mass of beaker 15 ml Initial volume reading Final volume reading Mass of beaker 20 ml 28.758 0.00 mL 5.00 mL 33.98 5.00 ml 10.05 ml 39.31 & 10.05 m 15.00 ml 49.45 15.00 ml 20.02 ml 49.81 28.618 0.00 ml 5.00 mL 32.56 5.00 mL 10.00 mL Ethanol Mass of empty beaker Initial volume reading Final volume reading Mass of beaker + 5 ml Initial volume reading Final volume reading Mass of beaker + 10 ml Initial volume reading Final volume reading Mass of beaker + 15 ml Initial volume reading Final volume reading Mass of beaker 20 ml 36.638 10.00 mL 15.00 ml 40.62 g 15.00 ml 20.00 mt. 44.63 -Seeking Regularities Name Danny & Calculations of Mass and Volume Quintal actual 5.-10.-"15," and "20 ml values based on the values in Table 1. In the event that we did not always get prechely the desired 5.00 10.00 15.00, and 20,00 volumes, we will determine the Table 2: Mass and Volume of Liquids Distilled water 15 Nafael Ethanol 5 ml mass B 5 ml mass 5 ml mass 5 ml volume ml 5 ml volume 15.00ml 5 ml volume 10 ml mass 8 8 10 ml mass 10.56 10 ml mass 10 ml volume mt 10 ml volume IDOSmt. ml 10 ml volume 15 ml mass 15 ml mass 15 ml mass 15 ml volume ml 15 mL volume mL 15 ml. volume ml 20 ml mass 20 ml mass 20 ml mass 20 ml volume ml ml 20 ml volume ml 20 ml volume ml The "5 ml mass is the mass of beaker + 5 ml)-(mass of empty beaker); similarly, the 10 ml massis (mass of beaker + 10ml)-(mass of empty beaker), and so on. Both mass and volume should increase in regular fashion All volumes and all masses are to be recorded to two decimal places. 33.98-28.75 -5.23g (5 ml) 39.31 - 28.75= 10.569 (10mL) C. Determination of Densities of the Three Liquids The ratio of mass to volume for a pure substance is called its density. Density is considered a "characteristic property of pure substances; by that we mean that the same substance always has the same mass-to-volume ratio- the same density. Your goal is to demonstrate that consistency for each of the three liquids. You will do so by calculating the density for each of the four volumes (5 ml, 10 ml, 15 ml, and 20 ml). Thus the '5 ml density column in the table below is found by dividing your 5 ml mass by your 5 ml volume. You will also calculate an average density for each of the three liquids. Complete the Density Table Show all of the density calculations for distilled water in the space below, then enter your results in the table at the top of the next page periment 2