Answered step by step
Verified Expert Solution
Question
1 Approved Answer
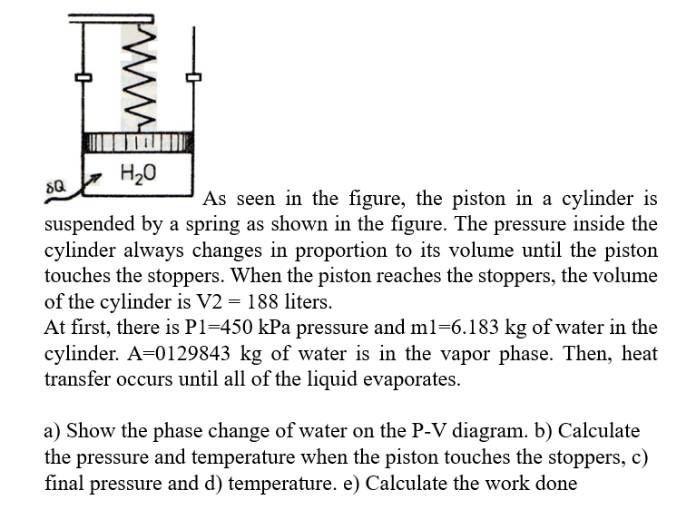
. . . seen in the figure, the piston in a cylinder is suspended by a spring as shown in the figure. The pressure inside
seen in the figure, the piston in a cylinder is
suspended by a spring as shown in the figure. The pressure inside the
cylinder always changes in proportion to its volume until the piston
touches the stoppers. When the piston reaches the stoppers, the volume
of the cylinder is V liters.
At first, there is kPa pressure and of water in the
cylinder. A of water is in the vapor phase. Then, heat
transfer occurs until all of the liquid evaporates.
a Show the phase change of water on the PV diagram. b Calculate
the pressure and temperature when the piston touches the stoppers, c
final pressure and d temperature. e Calculate the work done
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


