Question
Select the best conjugate acid-base pairs for buffer solutionsat the given pH values, and answer the question about bufferpreparation for each case. Use a single
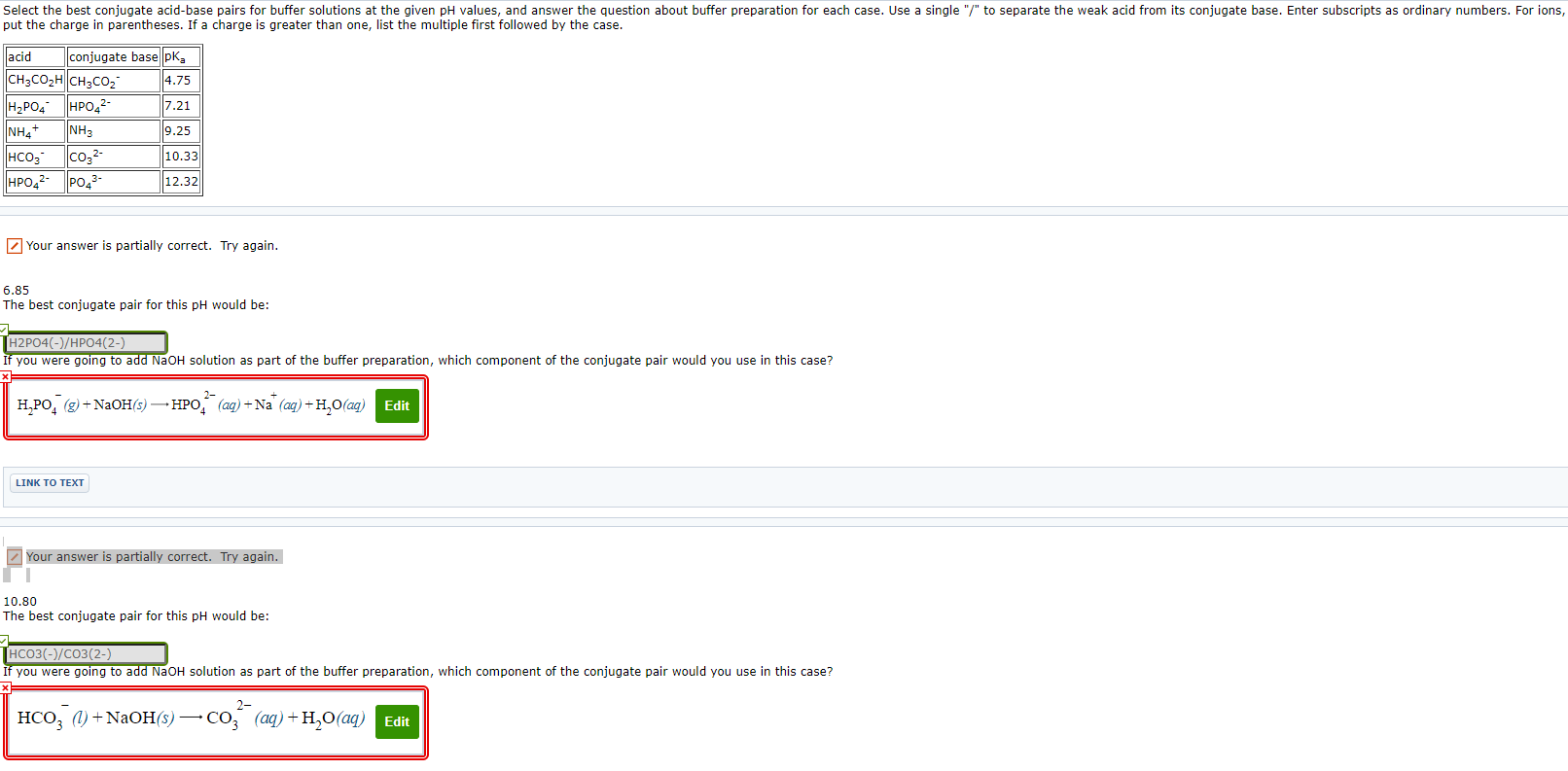
Select the best conjugate acid-base pairs for buffer solutionsat the given pH values, and answer the question about bufferpreparation for each case. Use a single "/" to separate the weakacid from its conjugate base. Enter subscripts as ordinary numbers.For ions, put the charge in parentheses. If a charge is greaterthan one, list the multiple first followed by the case.
| acid | conjugate base | pKa |
| CH3CO2H | CH3CO2- | 4.75 |
| H2PO4- | HPO42- | 7.21 |
| NH4+ | NH3 | 9.25 |
| HCO3- | CO32- | 10.33 |
| HPO42- | PO43- | 12.32 |
Part 1
6.85The best conjugate pair for this pH would be:
If you were going to add NaOH solution as part of the bufferpreparation, which component of the conjugate pair would you use inthis case?
H2PO4?(g)+NaOH(s)HPO42?(aq)+Na+(aq)+H2O(aq)
Part 2
10.80The best conjugate pair for this pH would be:
If you were going to add NaOH solution as part of the bufferpreparation, which component of the conjugate pair would you use inthis case?
Select the best conjugate acid-base pairs for buffer solutions at the given pH values, and answer the question about buffer preparation for each case. Use a single "/" to separate the weak acid from its conjugate base. Enter subscripts as ordinary numbers. For ions, put the charge in parentheses. If a charge is greater than one, list the multiple first followed by the case. acid conjugate base pka 4.75 7.21 9.25 10.33 12.32 CH3COH CH3CO HPO4 HPO42- NH4+ NH3 HCO co- HPO4- PO4- Your answer is partially correct. Try again. 6.85 The best conjugate pair for this pH would be: TH2PO4(-)/HPO4(2-) If you were going to add NaOH solution as part of the buffer preparation, which component of the conjugate pair would you use in this case? 2- HPO(g) + NaOH(s) HPO (aq) + Na (aq) + HO (ag) Edit LINK TO TEXT Your answer is partially correct. Try again. I 10.80 The best conjugate pair for this pH would be: HCO3(-)/CO3(2-) If you were going to add NaOH solution as part of the buffer preparation, which component of the conjugate pair would you use in this case? 2- HCO3 (1) + NaOH(s) CO (aq) + HO (aq) Edit
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
The best choice of buffer is that one which satisfies the condition below conjugate base acid pHpK l...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


