Answered step by step
Verified Expert Solution
Question
1 Approved Answer
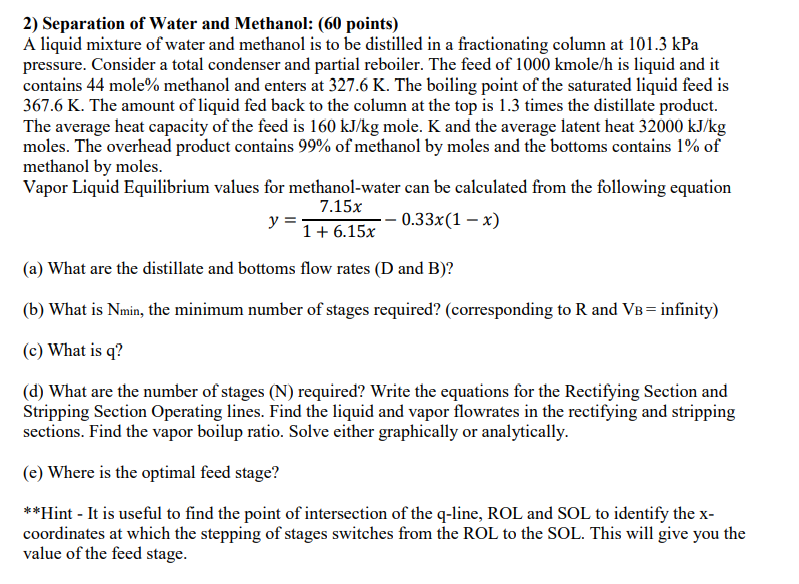
Separation of Water and Methanol: ( 6 0 points ) A liquid mixture of water and methanol is to be distilled in a fractionating column
Separation of Water and Methanol: points
A liquid mixture of water and methanol is to be distilled in a fractionating column at kPa
pressure. Consider a total condenser and partial reboiler. The feed of kmol is liquid and it
contains mole methanol and enters at The boiling point of the saturated liquid feed is
The amount of liquid fed back to the column at the top is times the distillate product.
The average heat capacity of the feed is mole. and the average latent heat
moles. The overhead product contains of methanol by moles and the bottoms contains of
methanol by moles.
Vapor Liquid Equilibrium values for methanolwater can be calculated from the following equation
a What are the distillate and bottoms flow rates D and B
b What is the minimum number of stages required? corresponding to and infinity
c What is q
d What are the number of stages N required? Write the equations for the Rectifying Section and
Stripping Section Operating lines. Find the liquid and vapor flowrates in the rectifying and stripping
sections. Find the vapor boilup ratio. Solve either graphically or analytically.
e Where is the optimal feed stage?
Hint It is useful to find the point of intersection of the qline, ROL and SOL to identify the x
coordinates at which the stepping of stages switches from the ROL to the SOL. This will give you the
value of the feed stage.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


