Question: Separations Inc. (SI) operates a three-stage extraction unit to separate 100 kg/min of a mixture containing a 50:50 (wt basis) mixture of benzene and hexane.

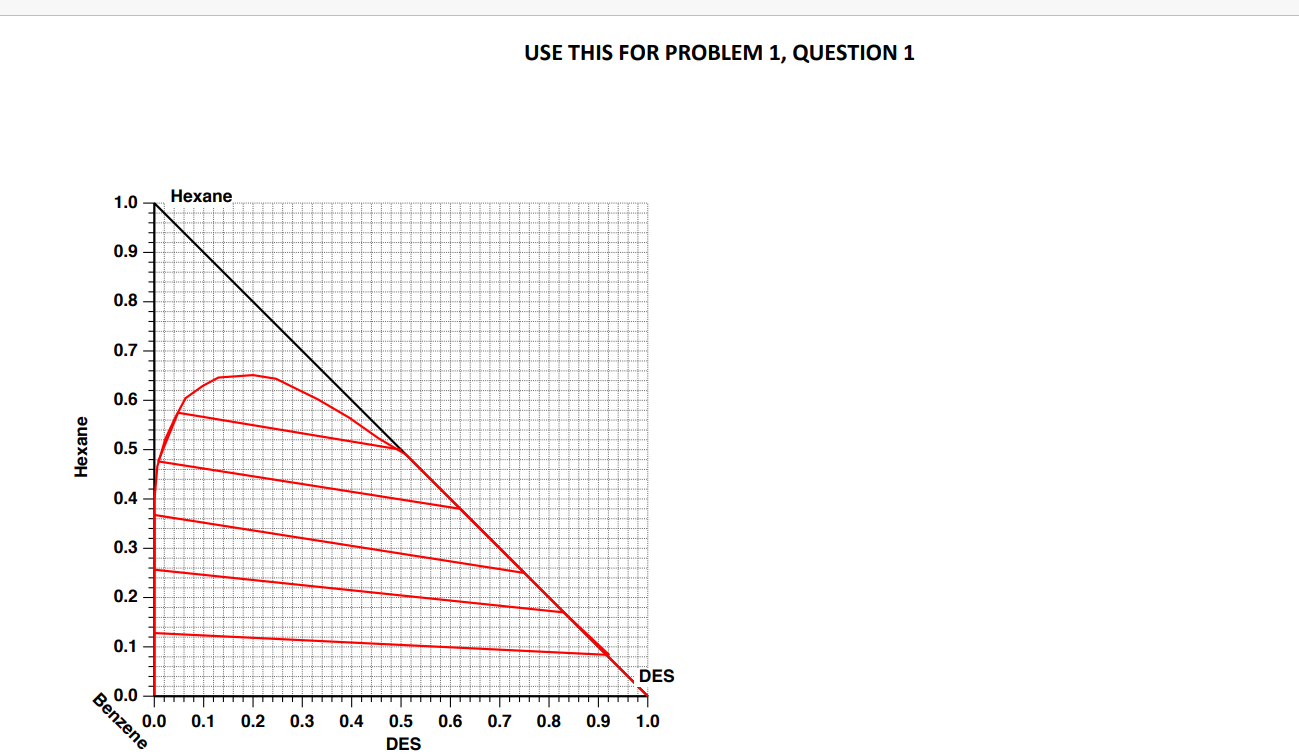
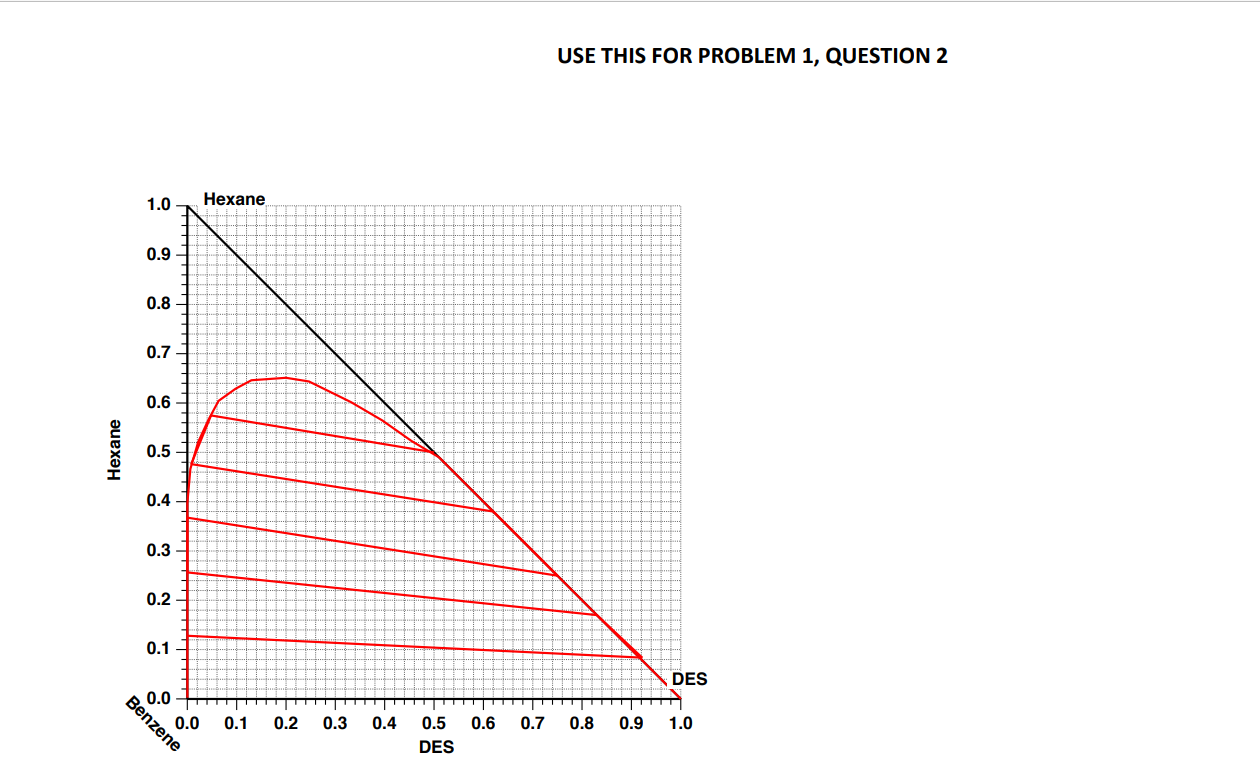
Separations Inc. (SI) operates a three-stage extraction unit to separate 100 kg/min of a mixture containing a 50:50 (wt basis) mixture of benzene and hexane. For this purpose, they use 145 kg/min of a proprietary solvent that results in a raffinate product containing 15 wt% hexane and 85% benzene on a solvent-free basis. There is a considerable amount of solvent present in both the extract and raffinate streams. The extract and raffinate streams are taken to distillation columns to separate the solvent that is then recycled. The solvent has moderately low volatility compared to hexane and benzene Your company has developed a new solvent called Deep-eutectic solvent (DES), and you believe that you can outperform the existing solvent used by SI. Your manager has tasked you to perform a compelling case to present to Sl. You have the equilibrium curve from your lab that has been confirmed in the literature as attached. DES is also known to have very low volatility compared to hexane and benzene 1. If you run a single-stage extraction unit with 100 kg/min feed and 145 kg/min of the DES ping solvent, what will be the composition and flow rates of the extract and raffinate streams? Use the equilibrium diagram to show the location of all four streams (feed, solvent, extract and raffinate). [7 MARKS] 2. By performing suitable calculations, check if the DES solvent can outperform Si's current solvent while guaranteeing the same raffinate composition and without requiring any change to the existing infrastructure (number of stages, pumps etc.). [12 MARKS] 3. List the other technical merits of switching to the DES solvent compared to Si's existing solvent. You have to justify it with the available equilibrium diagram and calculations from the previous question. [6 MARKS] USE THIS FOR PROBLEM 1, QUESTION 1 1.0 Hexane 0.9 0.8 0.7 0.6 Hexane 0.5 0.4 0.3 0.2 0.1- DES 0.0 0.0 0.1 0.2 0.3 0.4 0.6 0.7 0.8 0.9 1.0 Benzene 0.5 DES USE THIS FOR PROBLEM 1, QUESTION 2 1.0 Hexane 0.9 0.8 0.7 0.6 Hexane 0.5 0.4 0.3 0.2 0.1 DES Benzene 0.0 0.0 0.1 0.2 0.3 0.4 0.6 0.7 0.8 0.9 1.0 0.5 DES Separations Inc. (SI) operates a three-stage extraction unit to separate 100 kg/min of a mixture containing a 50:50 (wt basis) mixture of benzene and hexane. For this purpose, they use 145 kg/min of a proprietary solvent that results in a raffinate product containing 15 wt% hexane and 85% benzene on a solvent-free basis. There is a considerable amount of solvent present in both the extract and raffinate streams. The extract and raffinate streams are taken to distillation columns to separate the solvent that is then recycled. The solvent has moderately low volatility compared to hexane and benzene Your company has developed a new solvent called Deep-eutectic solvent (DES), and you believe that you can outperform the existing solvent used by SI. Your manager has tasked you to perform a compelling case to present to Sl. You have the equilibrium curve from your lab that has been confirmed in the literature as attached. DES is also known to have very low volatility compared to hexane and benzene 1. If you run a single-stage extraction unit with 100 kg/min feed and 145 kg/min of the DES ping solvent, what will be the composition and flow rates of the extract and raffinate streams? Use the equilibrium diagram to show the location of all four streams (feed, solvent, extract and raffinate). [7 MARKS] 2. By performing suitable calculations, check if the DES solvent can outperform Si's current solvent while guaranteeing the same raffinate composition and without requiring any change to the existing infrastructure (number of stages, pumps etc.). [12 MARKS] 3. List the other technical merits of switching to the DES solvent compared to Si's existing solvent. You have to justify it with the available equilibrium diagram and calculations from the previous question. [6 MARKS] USE THIS FOR PROBLEM 1, QUESTION 1 1.0 Hexane 0.9 0.8 0.7 0.6 Hexane 0.5 0.4 0.3 0.2 0.1- DES 0.0 0.0 0.1 0.2 0.3 0.4 0.6 0.7 0.8 0.9 1.0 Benzene 0.5 DES USE THIS FOR PROBLEM 1, QUESTION 2 1.0 Hexane 0.9 0.8 0.7 0.6 Hexane 0.5 0.4 0.3 0.2 0.1 DES Benzene 0.0 0.0 0.1 0.2 0.3 0.4 0.6 0.7 0.8 0.9 1.0 0.5 DES
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


