Answered step by step
Verified Expert Solution
Question
1 Approved Answer
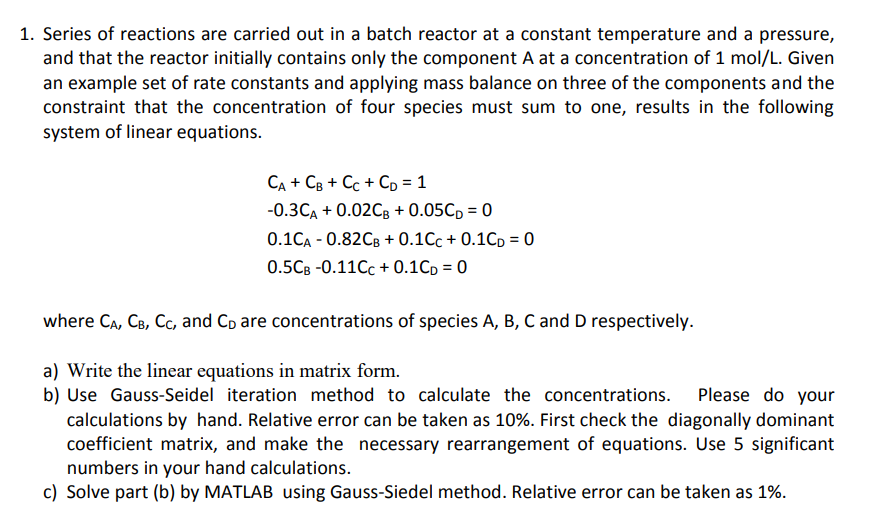
Series of reactions are carried out in a batch reactor at a constant temperature and a pressure, and that the reactor initially contains only the
Series of reactions are carried out in a batch reactor at a constant temperature and a pressure,
and that the reactor initially contains only the component at a concentration of Given
an example set of rate constants and applying mass balance on three of the components and the
constraint that the concentration of four species must sum to one, results in the following
system of linear equations.
where and are concentrations of species and respectively.
a Write the linear equations in matrix form.
b Use GaussSeidel iteration method to calculate the concentrations. Please do your
calculations by hand. Relative error can be taken as First check the diagonally dominant
coefficient matrix, and make the necessary rearrangement of equations. Use significant
numbers in your hand calculations.
c Solve part b by MATLAB using GaussSiedel method. Relative error can be taken as I need the MATLAB CODE for solve part
b
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


