Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Shen and Smith [Ind. Eng. Chem. Fundam., 7, 100- 105 (1968)] measured equilibrium-adsorption isotherms at four different temperatures for pure benzene vapor on silica
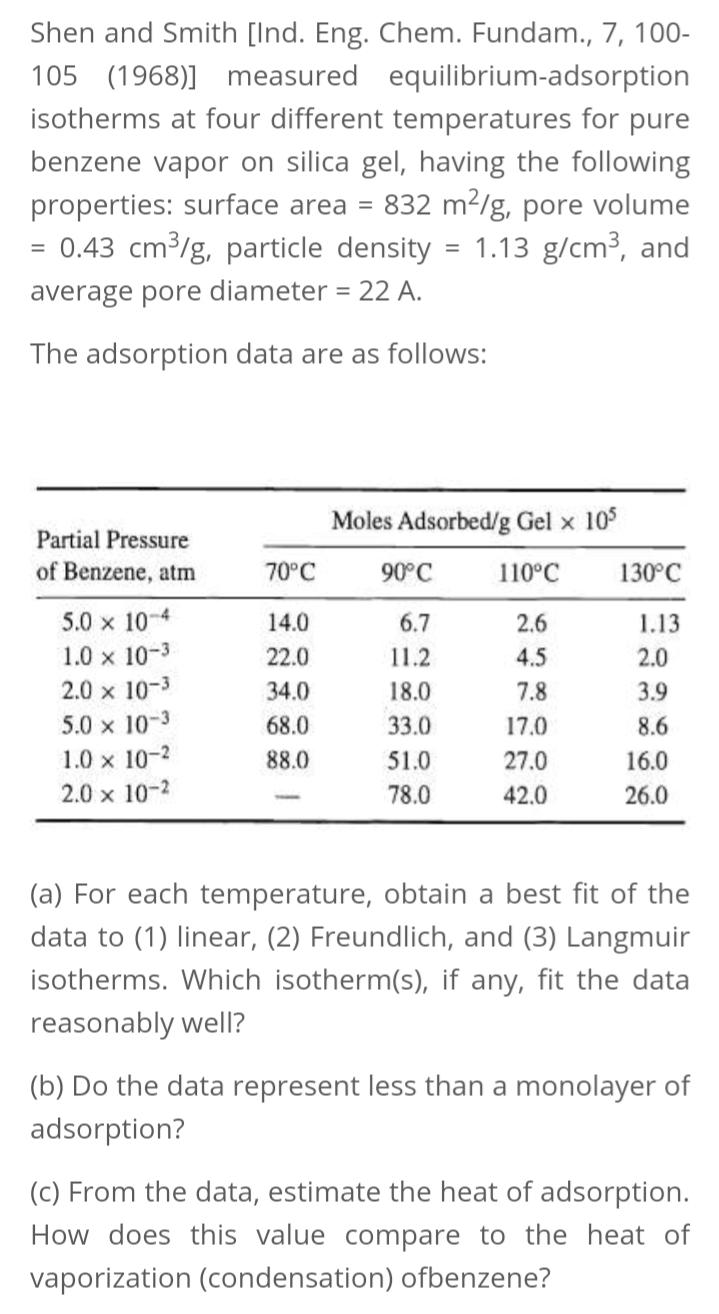
Shen and Smith [Ind. Eng. Chem. Fundam., 7, 100- 105 (1968)] measured equilibrium-adsorption isotherms at four different temperatures for pure benzene vapor on silica gel, having the following properties: surface area = 832 m2/g, pore volume = 0.43 cm/g, particle density = 1.13 g/cm3, and average pore diameter = 22 A. The adsorption data are as follows: Moles Adsorbed/g Gel x 10 Partial Pressure of Benzene, atm 70C 90C 110C 130C 5.0 x 10-4 14.0 6.7 2.6 1.13 1.0 x 10-3 2.0 x 10-3 22.0 11.2 4.5 2.0 34.0 18.0 7.8 3.9 5.0 x 10-3 1.0 x 10-2 68.0 33.0 17.0 8.6 88.0 51.0 27.0 16.0 2.0 x 10-2 78.0 42.0 26.0 (a) For each temperature, obtain a best fit of the data to (1) linear, (2) Freundlich, and (3) Langmuir isotherms. Which isotherm(s), if any, fit the data reasonably well? (b) Do the data represent less than a monolayer of adsorption? (c) From the data, estimate the heat of adsorption. How does this value compare to the heat of vaporization (condensation) ofbenzene?
Step by Step Solution
★★★★★
3.50 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
According to the given dat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


