Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show me steps please From the following spectrophotometer test results of a 1.00cm (path length) sample that has an absorbance of 0.64. Answer the following
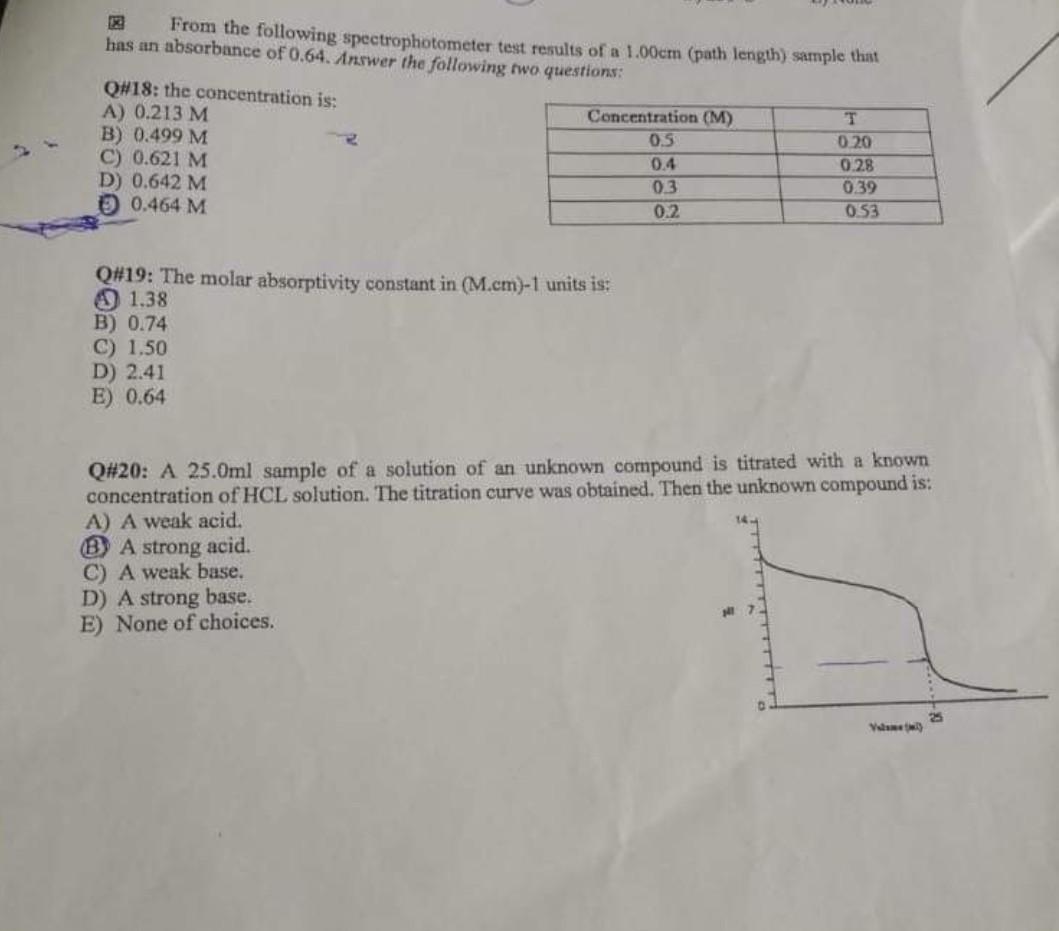
show me steps please
From the following spectrophotometer test results of a 1.00cm (path length) sample that has an absorbance of 0.64. Answer the following two questions: Q#18: the concentration is: Concentration (M) T A) 0.213 M B) 0.499 M C) 0.621 M 0.5 0.20 0.4 0.28 D) 0.642 M 0.3 0.39 0.464 M 0.2 0.53 Q#19: The molar absorptivity constant in (M.cm)-1 units is: 1.38 B) 0.74 C) 1.50 D) 2.41 E) 0.64 Q#20: A 25.0ml sample of a solution of an unknown compound is titrated with a known concentration of HCL solution. The titration curve was obtained. Then the unknown compound is: A) A weak acid. BA strong acid. C) A weak base. D) A strong base. E) None of choices. 25 Valame()Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


