Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show procedure Score on last try: 0.75 of 1 pts. See Details for more. > Next question You can retry this question below The fizz
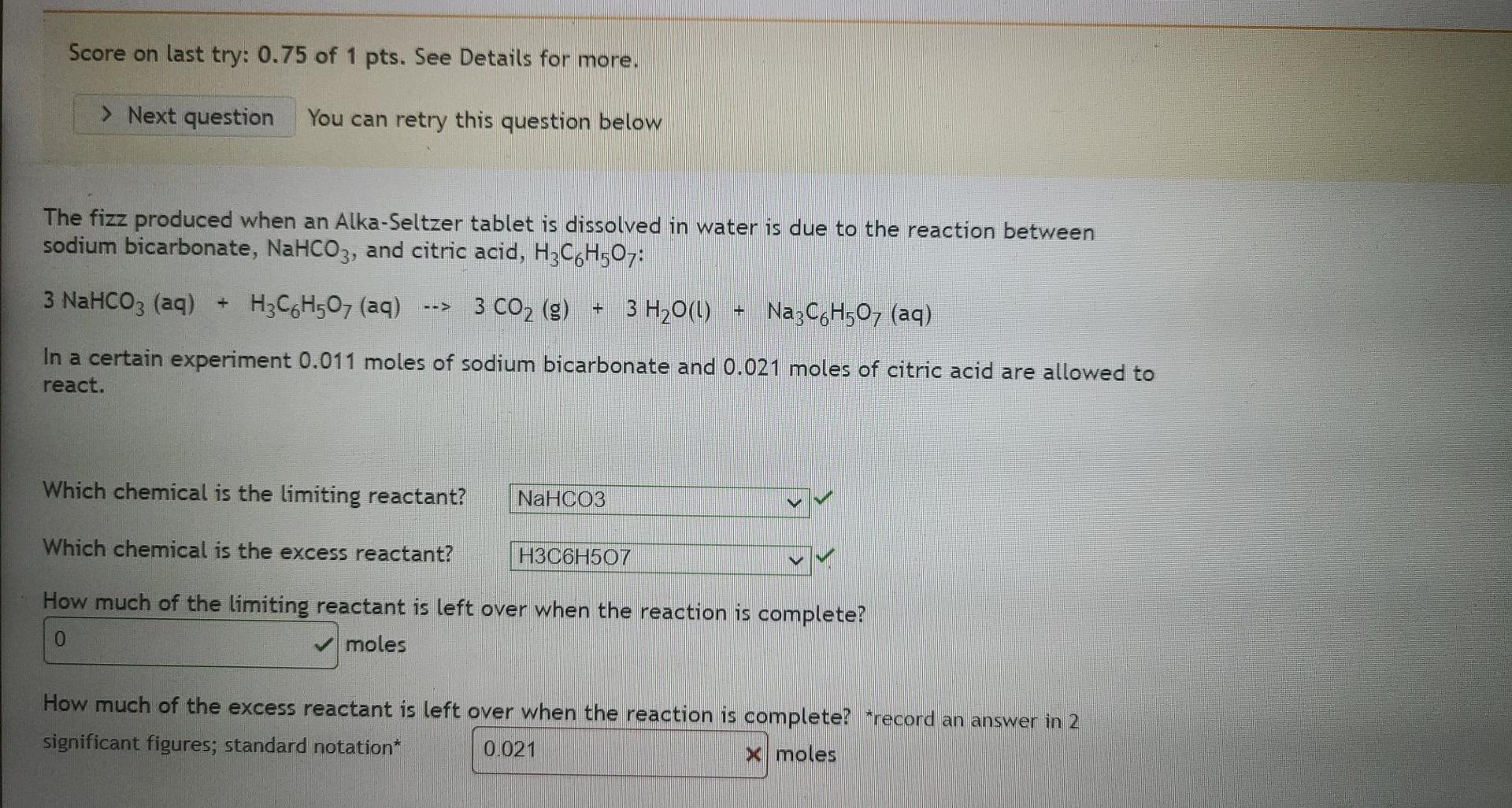
show procedure
Score on last try: 0.75 of 1 pts. See Details for more. > Next question You can retry this question below The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction between sodium bicarbonate, NaHCO3, and citric acid, H3C6H507: 3 NaHCO3 (aq) + H3C6H507 (aq) --> 3 CO2 (g) + 3 H2O(l) + Na3C6H507 (aq) In a certain experiment 0.011 moles of sodium bicarbonate and 0.021 moles of citric acid are allowed to react. Which chemical is the limiting reactant? NaHCO3 Which chemical is the excess reactant? H3C6H507 How much of the limiting reactant is left over when the reaction is complete? moles 0 How much of the excess reactant is left over when the reaction is complete? *record an answer in 2 significant figures; standard notation* 0.021 X molesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


