Question
Show that the translational motion makes the greatest contribution to the internal energy and enthalpy and explain clearly why this is the case. .
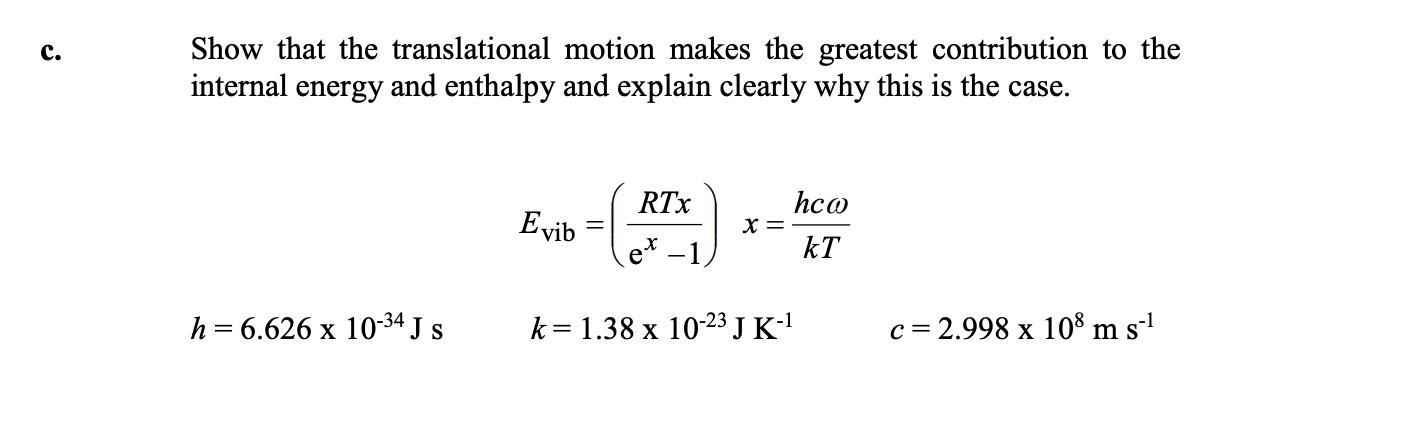
Show that the translational motion makes the greatest contribution to the internal energy and enthalpy and explain clearly why this is the case. . hco x = kT RTx Evib e* -1, h = 6.626 x 10-34 J s k= 1.38 x 10-23 J K-1 c = 2.998 x 10 m s-
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics for Engineers
Authors: Kenneth A. Kroos, Merle C. Potter
1st edition
1133112862, 978-113311286
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



