Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show work please 6) A galvanic cell is designated by: BeBe, 0.25M) || NO: (ay, 0.75M), H(a,0.5M) | NOC, 0.25M) a) Write each of the
show work please 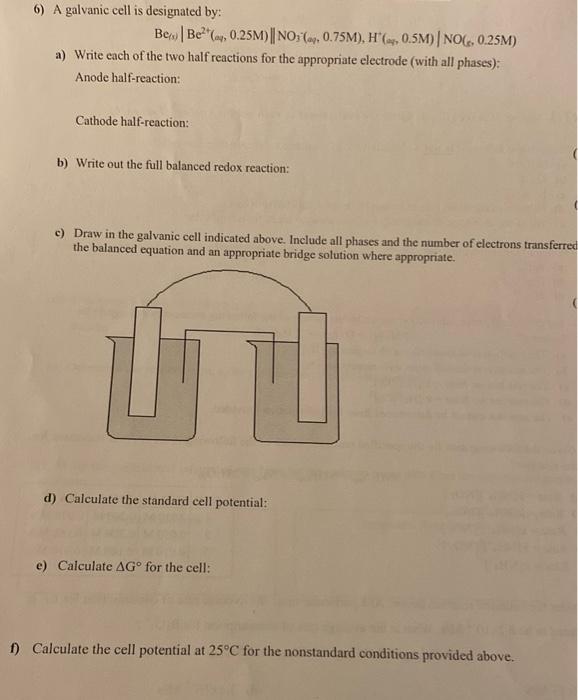
6) A galvanic cell is designated by: BeBe, 0.25M) || NO: (ay, 0.75M), H(a,0.5M) | NOC, 0.25M) a) Write each of the two half reactions for the appropriate electrode (with all phases): Anode half-reaction: Cathode half-reaction: b) Write out the full balanced redox reaction: c) Draw in the galvanic cell indicated above. Include all phases and the number of electrons transferred the balanced equation and an appropriate bridge solution where appropriate 1 d) Calculate the standard cell potential: e) Calculate AG for the cell: 1) Calculate the cell potential at 25C for the nonstandard conditions provided above 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


