Question: Various Gas Expansions: pV Plots and Work An ideal monatomic gas is contained in a cylinder with a movable piston so that the gas
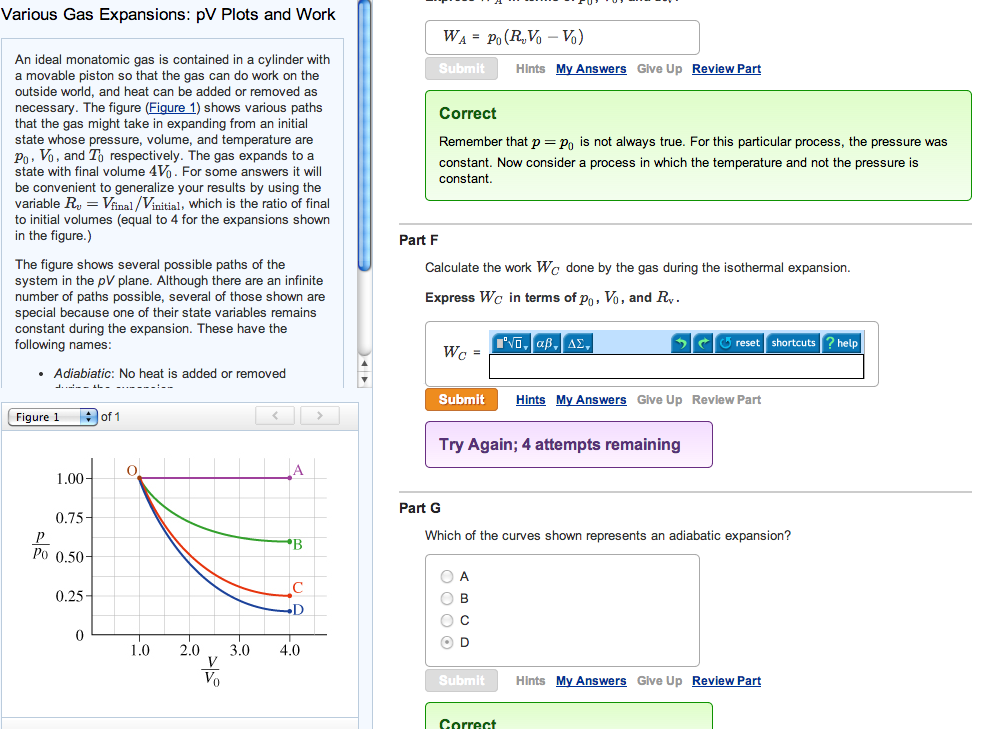
Various Gas Expansions: pV Plots and Work An ideal monatomic gas is contained in a cylinder with a movable piston so that the gas can do work on the outside world, and heat can be added or removed as necessary. The figure (Figure 1) shows various paths that the gas might take in expanding from an initial state whose pressure, volume, and temperature are Vo, and To respectively. The gas expands to a state with final volume 4V. For some answers it will be convenient to generalize your results by using the variable R = Vfinal/Vinitial, which is the ratio of final to initial volumes (equal to 4 for the expansions shown in the figure.) The figure shows several possible paths of the system in the pV plane. Although there are an infinite number of paths possible, several of those shown are special because one of their state variables remains constant during the expansion. These have the following names: Adiabiatic: No heat is added or removed Figure 1 1.00- 0.75- Po 0.50- 0.25- 0 - of 1 O 1.0 2.0 D 3.0 4.0 WA PO (RVo - Vo) Submit Correct Remember that p = Po is not always true. For this particular process, the pressure was constant. Now consider a process in which the temperature and not the pressure is constant. Part F Calculate the work We done by the gas during the isothermal expansion. Express Wc in terms of po, Vo, and R. , , , 5 reset shortcuts ? help Wc Submit Hints My Answers Give Up Review Part Try Again; 4 attempts remaining O A O C OD Part G Which of the curves shown represents an adiabatic expansion? Submit Hints My Answers Give Up Review Part Correct Hints My Answers Give Up Review Part
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Solution Part F Work done in isothermal process is given by W c nRT ln V f ... View full answer

Get step-by-step solutions from verified subject matter experts


