Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show work please:) Problem 2. Distillation with multiple units (60 points) One hundred kilomoles per hour of a liquid mixture (the process feed) containing 30.0
show work please:) 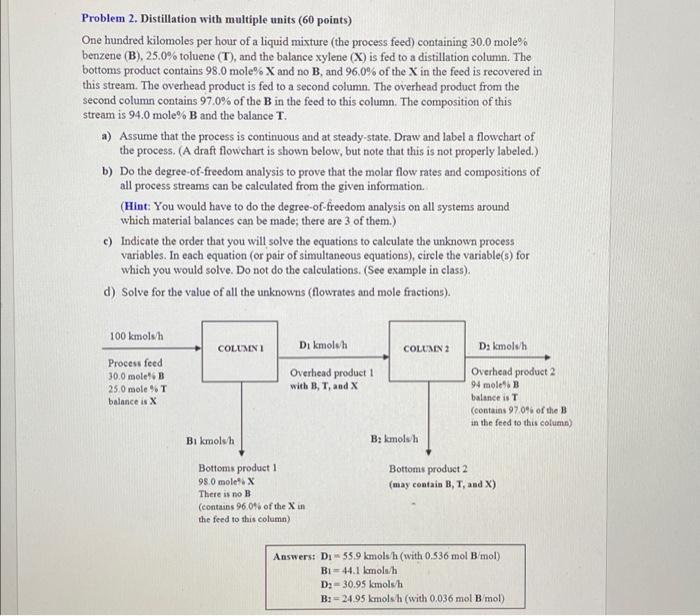
Problem 2. Distillation with multiple units (60 points) One hundred kilomoles per hour of a liquid mixture (the process feed) containing 30.0 mole% benzene (B), 25.0% tolueno (T), and the balance xylene (X) is fed to a distillation column. The bottoms product contains 98.0 mole% X and no B, and 96.0% of the X in the feed is recovered in this stream. The overhead product is fed to a second column. The overhead product from the second column contains 97.0% of the B in the feed to this column. The composition of this stream is 94.0 mole% B and the balance T. a) Assume that the process is continuous and at steady-state, Draw and label a flowchart of the process. (A draft flowchart is shown below, but note that this is not properly labeled.) b) Do the degree-of-freedom analysis to prove that the molar flow rates and compositions of all process streams can be calculated from the given information (Hint: You would have to do the degree-of-freedom analysis on all systems around which material balances can be made there are 3 of them.) c) Indicate the order that you will solve the equations to calculate the unknown process variables. In each equation (or pair of simultaneous equations), circle the variable(s) for which you would solve. Do not do the calculations. (See example in class). d) Solve for the value of all the unknowns (flowrates and mole fractions). 100 kmolsh COLUMN Di kmolah COLUMN 2 Dakmolsh Process feed 30.0 molet. B 25.0 mole %T balance is X Overhead product 1 with B, T, and X Overhead product 2 94 mole" B balance is (contains 970% of the B in the feed to this column) B1 kmolsh B: kamolsh Bottoms product 2 (may contain B, T, and x) Bottoms product 1 98.0 mole'. X There is no B (contains 96.0% of the Xin the feed to this column) Answers: Di55.9 kmols/h (with 0.536 mol B/mol) Bi-44.1 kmolsh Da 30.95 kmols/h B:= 24.95 kmolsh (with 0.036 mol B mol) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


