Question
Chemical Equations Instructions: Write the word equations for the chemical equations below and determine the type of reaction (synthesis/combination, decomposition, single-displacemet, double-displacement, or combustion).
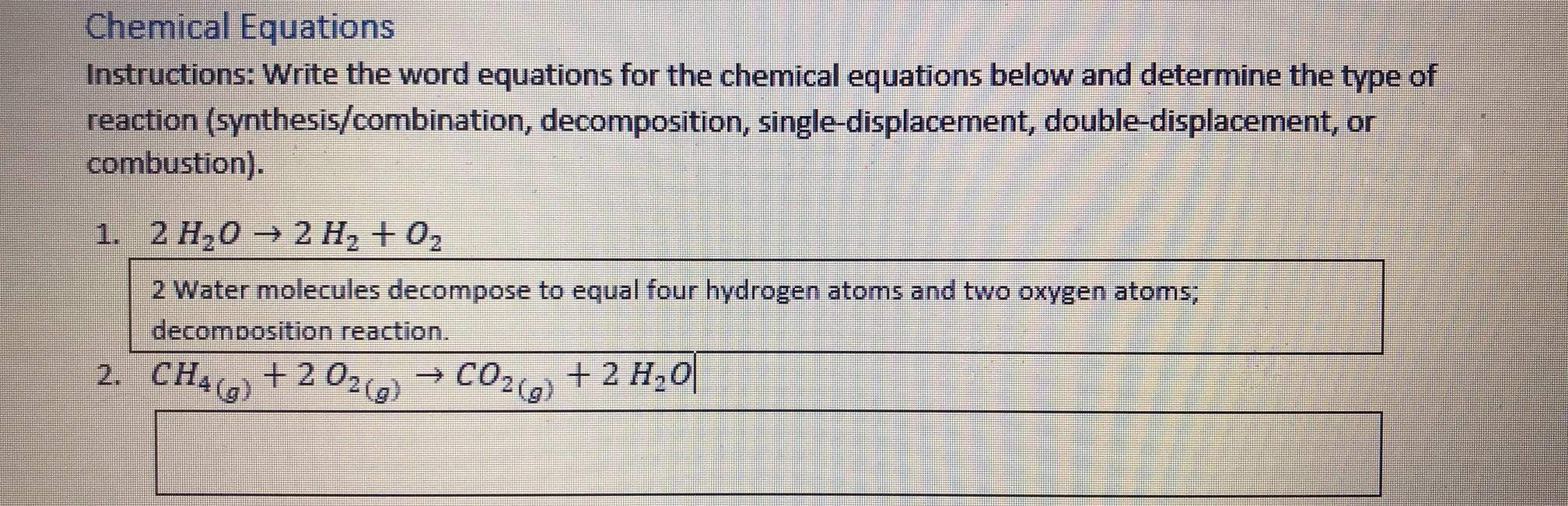
Chemical Equations Instructions: Write the word equations for the chemical equations below and determine the type of reaction (synthesis/combination, decomposition, single-displacemet, double-displacement, or combustion). 1. 2 -0 2 , + 2 2 Water molecules decompose to equal four hydrogen atoms and two oxygen atoms; decomposition reaction. 2. CHAG) + 2 02 CO2) + 2 H,0 ->
Step by Step Solution
3.51 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
1 Word equation Water Hydrogen Oxygen Type of reaction The reaction is Decomposi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Francis A. Carey
4th edition
0072905018, 978-0072905014
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



