
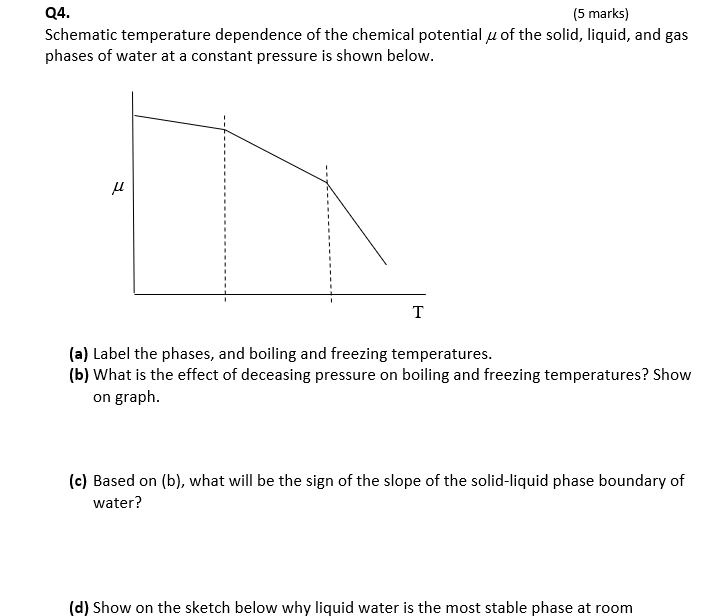
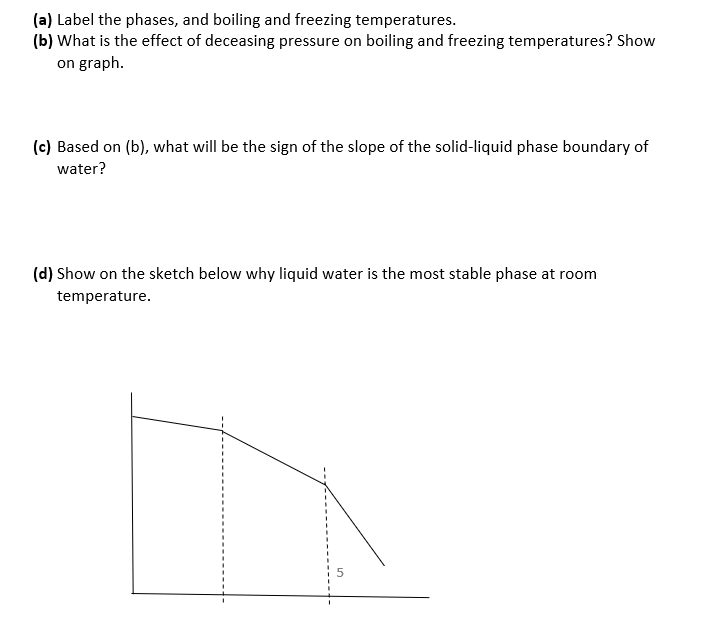

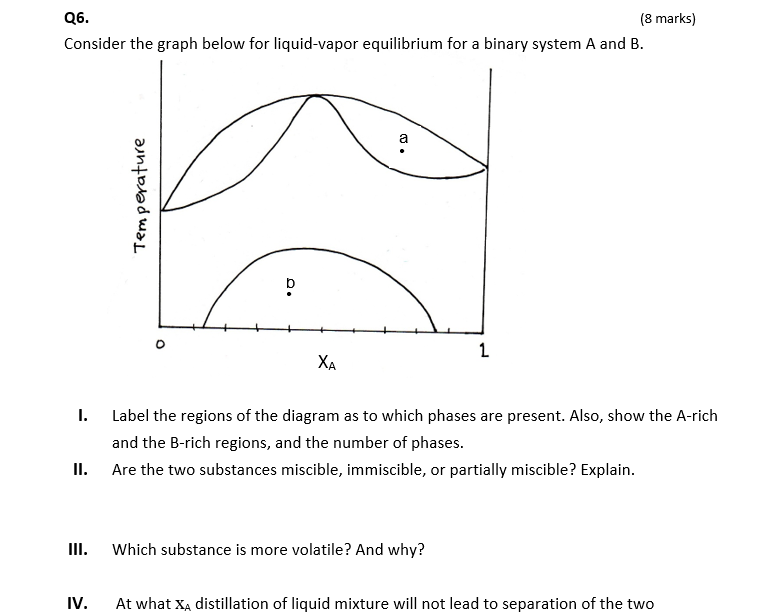


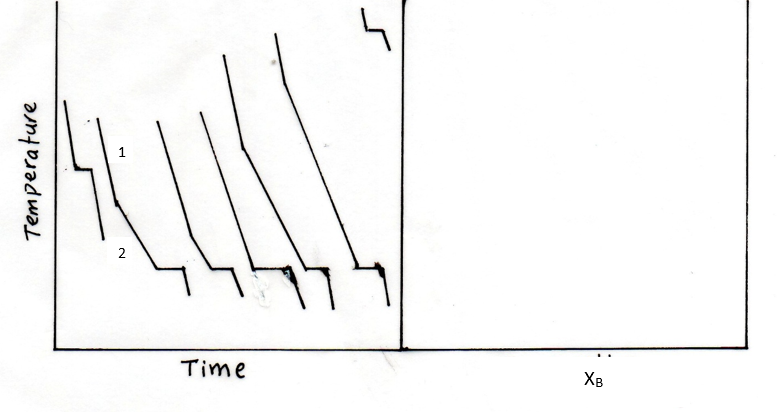
Show your work as much. Derive all relations you use unless they appear in front page Q1. (8 marks) 1.65 mol of a perfect gas for which Cv,m = 12.47 J K-1 mol is subjected to two successive changes in state: (1) from 37.0 C and 1.00x105 Pa, the gas is expended isothermally against a constant pressure of 16.5x103 Pa to twice its initial volume. (2) At the end of the previous process, the gas is cooled at constant volume from 37.0C to - 23.0 C. (a) Calculate q,w, AU, AH for each of the stages. (b) Construct P-V diagram for the two processes. P V Q2. (4 marks) Calculate AS, AS surr, AS total when the volume of 4.39 mol of CO gas initially at 298 K and 1.00 bar increases by a factor of four in (a) Adiabatic reversible expansion. (b) Isothermal reversible expansion. Tsurr = 298 K. State whether each process is spontaneous. Q3. (5 marks) When 0.865 g sample of cyclohexane (molar mass = 84.16 g/mol) is burned in a bomb calorimeter, the temperature of the inner water bath and the calorimeter increases by 2.76 K. If Aue combustion of cyclohexane is 3913 kJ/mol and the mass of the water in the inner bath is 1820 g, what is the calorimeter constant? Cp,m of water is 75.3 J K-1 mol-2. Recall that heat released by combustion is absorbed by both the inner water bath and the calorimeter. Q4. (5 marks) Schematic temperature dependence of the chemical potential u of the solid, liquid, and gas phases of water at a constant pressure is shown below. u T (a) Label the phases, and boiling and freezing temperatures. (b) What is the effect of deceasing pressure on boiling and freezing temperatures? Show on graph. (c) Based on (b), what will be the sign of the slope of the solid-liquid phase boundary of water? (d) Show on the sketch below why liquid water is the most stable phase at room (a) Label the phases, and boiling and freezing temperatures. (b) What is the effect of deceasing pressure on boiling and freezing temperatures? Show on graph. (c) Based on (b), what will be the sign of the slope of the solid-liquid phase boundary of water? (d) Show on the sketch below why liquid water is the most stable phase at room temperature. Q5. (5 marks) A 5.40 g of a substance dissolved in 135 g of CCl4 leads to an elevation of the boiling point of 0.650 C. Kp = 30 K kg mol-1 and Kb = 4.95 K kg mol-1 Calculate the freezing point depression, the molar mass of the substance, and of CCl4. demo Q6. (8 marks) Consider the graph below for liquid-vapor equilibrium for a binary system A and B. a Temperature b XA I. Label the regions of the diagram as to which phases are present. Also, show the A-rich and the B-rich regions, and the number of phases. Are the two substances miscible, immiscible, or partially miscible? Explain. II. Which substance is more volatile? And why? IV. At what XA distillation of liquid mixture will not lead to separation of the two v. At point a, what is the mole fraction of A in the vapor that is in equilibrium with liquid? VI. At what temperature will a mixture with XA = 0.3 start to evaporate? Show on graph. VII. If vapor at temperature of part VI is removed and condensed elsewhere, will XA increase or decrease? And why? VIII. What is the ratio of A-rich region to the B-rich region at point b? Q7. (5 marks) (a) Based on the cooling curves below, construct the temperature-composition phase diagram of two immiscible solids A and B and their miscible liquids. (b) Label the regions of the diagram as to which phases are present. (c) Consider the second cooling curve to the left, why the cooling rate is lower in path 2 compared to path 1? 5 Temperature Time XB
















