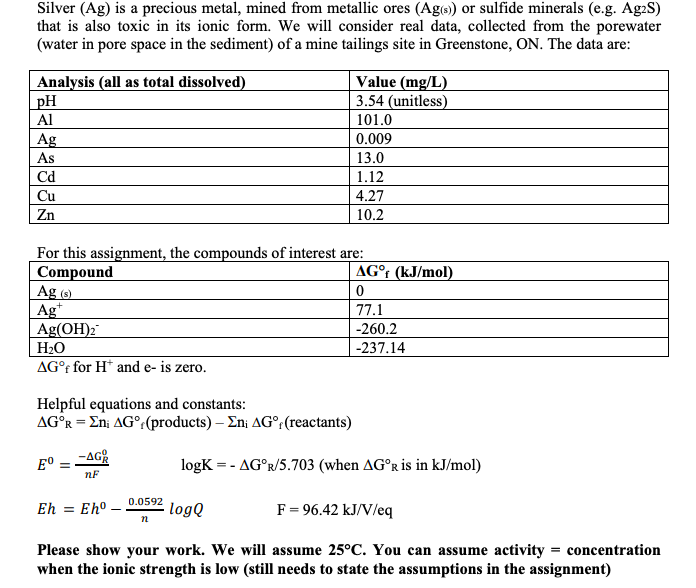

Silver (Ag) is a precious metal, mined from metallic ores (Ag(s) or sulfide minerals (e.g. Ag2S) that is also toxic in its ionic form. We will consider real data, collected from the porewater (water in pore space in the sediment) of a mine tailings site in Greenstone, ON. The data are: Analysis (all as total dissolved) pH Al Ag As Value (mg/L) 3.54 (unitless) 101.0 0.009 13.0 1.12 4.27 10.2 cd Cu Zn For this assignment, the compounds of interest are: Compound AG (kJ/mol) Ag (8) 0 Ag 77.1 Ag(OH)2 -260.2 H2O -237.14 AGf for H and e- is zero. Helpful equations and constants: AGR = En; AG (products) - En; AGr(reactants) E -AGR logK =- AGr/5.703 (when AGr is in kJ/mol) nF Eh = Eh - 0.0592 logQ F = 96.42 kJ/V/eq n Please show your work. We will assume 25C. You can assume activity = concentration when the ionic strength is low (still needs to state the assumptions in the assignment) 8. If the mine tailings are intruded by seawater. Assuming the ionic strength of porewater increases to 0.8 M due to the intrusion of seawater. Under this new condition, how is the equilibrium line drawn in Question 4e changed? Show your work and draw a new line to your graph. (3 marks) 9. Under standard conditions, which ion(s) could you add to the mine tailing pond to reduce dissolved Ag to Ag(s), thus removing the Ag+ contamination? explain by showing the redox reaction between the added ion(s) and Agt. Please show your work. Hint: Using the table of standard electrode potential values in the notes to find out which ion can reduce Agt but not harmful to the environment. It is because you do not want to remediate the contamination by introduction another contaminant into the mine tailing pond. (3 marks) Silver (Ag) is a precious metal, mined from metallic ores (Ag(s) or sulfide minerals (e.g. Ag2S) that is also toxic in its ionic form. We will consider real data, collected from the porewater (water in pore space in the sediment) of a mine tailings site in Greenstone, ON. The data are: Analysis (all as total dissolved) pH Al Ag As Value (mg/L) 3.54 (unitless) 101.0 0.009 13.0 1.12 4.27 10.2 cd Cu Zn For this assignment, the compounds of interest are: Compound AG (kJ/mol) Ag (8) 0 Ag 77.1 Ag(OH)2 -260.2 H2O -237.14 AGf for H and e- is zero. Helpful equations and constants: AGR = En; AG (products) - En; AGr(reactants) E -AGR logK =- AGr/5.703 (when AGr is in kJ/mol) nF Eh = Eh - 0.0592 logQ F = 96.42 kJ/V/eq n Please show your work. We will assume 25C. You can assume activity = concentration when the ionic strength is low (still needs to state the assumptions in the assignment) 8. If the mine tailings are intruded by seawater. Assuming the ionic strength of porewater increases to 0.8 M due to the intrusion of seawater. Under this new condition, how is the equilibrium line drawn in Question 4e changed? Show your work and draw a new line to your graph. (3 marks) 9. Under standard conditions, which ion(s) could you add to the mine tailing pond to reduce dissolved Ag to Ag(s), thus removing the Ag+ contamination? explain by showing the redox reaction between the added ion(s) and Agt. Please show your work. Hint: Using the table of standard electrode potential values in the notes to find out which ion can reduce Agt but not harmful to the environment. It is because you do not want to remediate the contamination by introduction another contaminant into the mine tailing pond








