Answered step by step
Verified Expert Solution
Question
1 Approved Answer
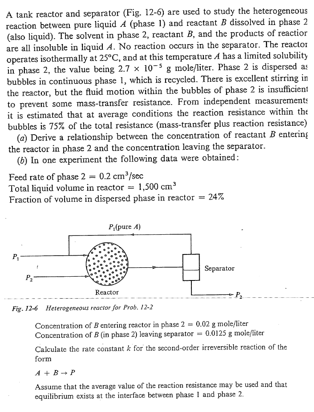
SMITH CHEMICAL ENGINEERING KINETICS. 1 2 - 2 A tank reactor and separator ( Fig . 1 2 - 6 ) are used to study
SMITH CHEMICAL ENGINEERING KINETICS.
A tank reactor and separator Fig are used to study the heterogeneous reaction between pure liquid phase and reactant dissolved in phase also liquid The solvent in phase reactant and the products of reactior are all insoluble in liquid No reaction occurs in the separator. The reacton operates isothermally at and at this temperature A has a limited solubility in phase the value being moleliter Phase is dispersed as bubbles in continuous phase which is recycled. There is excellent stirring in the reactor, but the tluid motion within the bubbles of phase is insufficient to prevent some masstransfer resistance. From independent measurements it is estimated that at average conditions the reaction resistance within the bubbles is of the total resistance masstransfer plus reaction resistance
a Derive a relationship between the concentration of reactant entering
the reactor in phase and the concentration leaving the separator.
b In one experiment the following data were obtained:
Feed rate of phase
Total liquid volume in reactor
Fraction of volume in dispersed phase in reactor
Conctentration of entering reactor ia phase moleliter
Concentration of in phase leaving irparator mole firer
Calculate the rave constant for the socondorder irreversible resction of the
form
Assume that the average value of the reaction resistance may be used and that
equilibrium exists at the interface betwetn plase and phase
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


