Answered step by step
Verified Expert Solution
Question
1 Approved Answer
SO2 is stored in a gasometer at a pressure of 1.2 at (abs) and 20C, from which 1 kg/s is transported to a fixed-bed reactor.
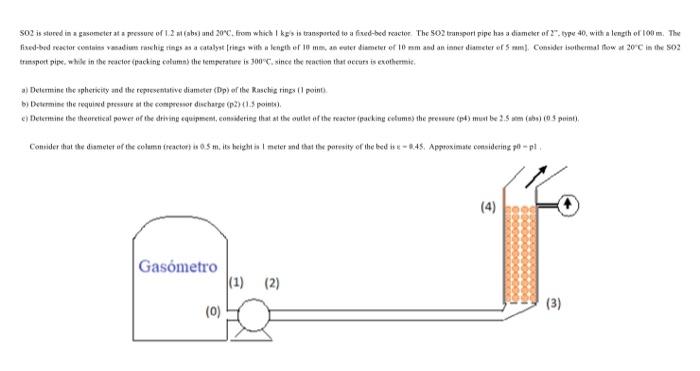
SO2 is stored in a gasometer at a pressure of 1.2 at (abs) and 20C, from which 1 kg/s is transported to a fixed-bed reactor. The SO2 transport pipe has a diameter of 2, type 40, with a length of 100 m. The fixed-bed reactor contains vanadium raschig rings as a catalyst [rings with a length of 10 mm, an outer diameter of 10 mm and an inner diameter of 5 mm]. Consider isothermal flow at 20C in the SO2 transport pipe, while in the reactor (packing column) the temperature is 300C, since the reaction that occurs is exothermic.
a) Determine the sphericity and the representative diameter (Dp) of the Raschig rings (1 point).
b) Determine the required pressure at the compressor discharge (p2) (1.5 points).
c) Determine the theoretical power of the driving equipment, considering that at the outlet of the reactor (packing column) the pressure (p4) must be 2.5 atm (abs) (0.5 point).
Consider that the diameter of the column (reactor) is 0.5 m, its height is 1 meter and that the porosity of the bed is = 0.45. Approximate considering p0 = p1 .
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


