Question
Sodium nitrite, NaNO, has a molar mass of 85.0 g/mol. What volume of 1.60 M NaNO; (aq) is needed in order to give 17.0
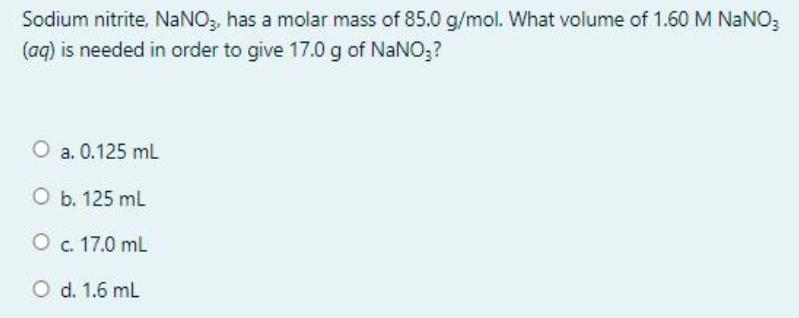
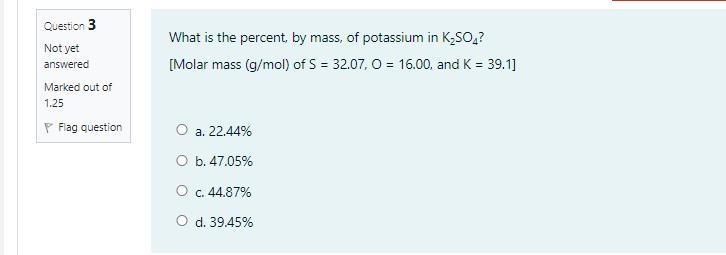
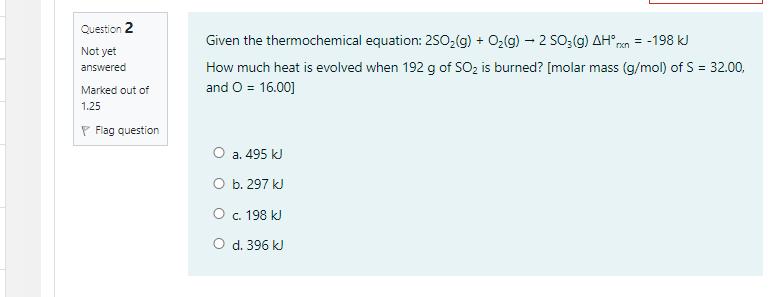
Sodium nitrite, NaNO, has a molar mass of 85.0 g/mol. What volume of 1.60 M NaNO; (aq) is needed in order to give 17.0 g of NaNO;? O a. 0.125 mL O b. 125 mL O c. 17.0 ml O d. 1.6 mL Question 3 What is the percent, by mass, of potassium in K;SO,? Not yet [Molar mass (g/mol) of S = 32.07, O = 16.00, and K = 39.1] answered Marked out of 1.25 P Flag question O a. 22.44% . O b. 47.05% O c. 44.87% O d. 39.45% Question 2 Given the thermochemical equation: 250,(g) + O2(g) 2 so;(g) AH ren = -198 kJ How much heat is evolved when 192 g of SOz is burned? [molar mass (g/mol) of S = 32.00, and O = 16.00] Not yet answered Marked out of 1.25 P Flag question . 495 k O b. 297 kJ . 198 k O d. 396 kJ
Step by Step Solution
3.33 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



