Question
Solid silicon in contact with solid silicon dioxide is to be heated to a temperature of 1100 K in a vacuum furnace. The two
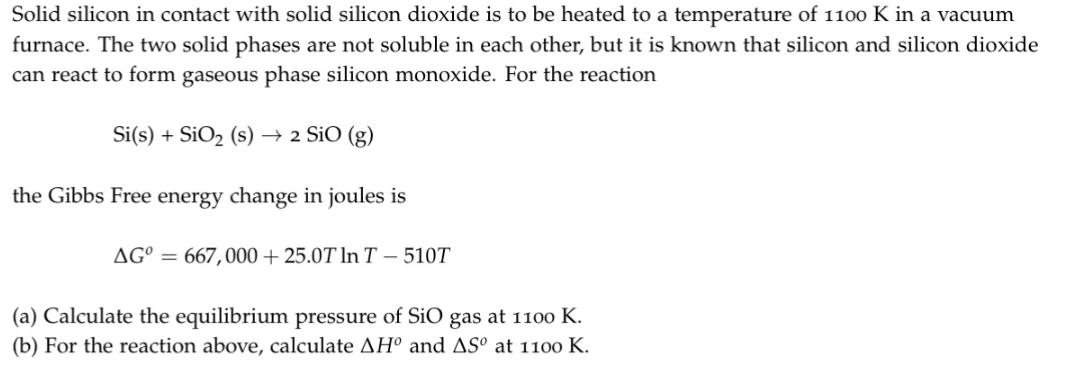
Solid silicon in contact with solid silicon dioxide is to be heated to a temperature of 1100 K in a vacuum furnace. The two solid phases are not soluble in each other, but it is known that silicon and silicon dioxide can react to form gaseous phase silicon monoxide. For the reaction Si(s) + SiO (s) 2 SiO (g) the Gibbs Free energy change in joules is AG = 667,000+25.0T In T- 510T (a) Calculate the equilibrium pressure of SiO gas at 1100 K. (b) For the reaction above, calculate AH and AS at 1100 K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a To calculate the equilibrium pressure of SiO gas at 1100 K we can use the Gibbs free energy change expression G RT ln Kp where Kp is the equilibrium constant for the reaction At equilibrium the chem...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Thermal-Fluid Sciences
Authors: Yunus A. Cengel, Robert H. Turner, John M. Cimbala
5th edition
78027680, 78027683, 9781760421359, 978-0078027680
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



