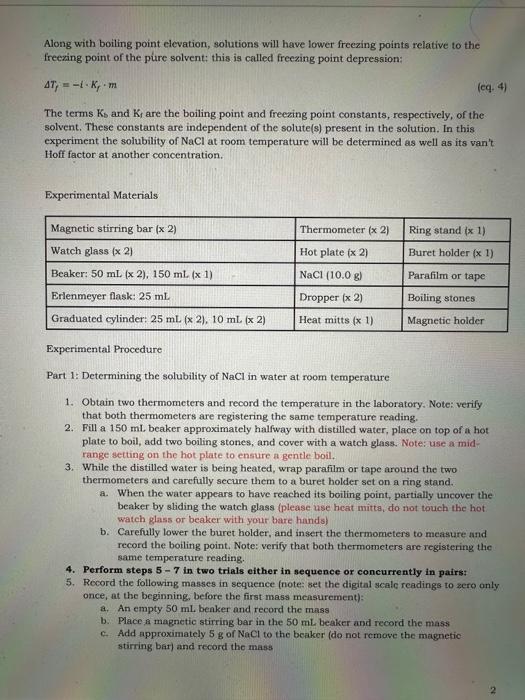
solubility =100mLofsolventmassofsolute(g) or mLofsolventmassofsolute(mg) or 1Lofsolutionmolesofsolute(M) Certain physical properties of liquid-phased solutions differ in specifie ways from those of the pure solvent and these differences depend only on how much solute is present. Properties of this type are called colligative properties and they include, but are not limited to, changes to boiling and freezing points, changes to vapor pressure, and changes to osmotic pressure. Boiling points of solutions, for instance, are higher than those of the pure solvent. This is called boiling point elevation and the change in boiling point can be determined using the following equation: Tb=iKbm In this equation Tb is the difference in boiling point of the solution relative to that of the pure solvent. Tb=( (bolling point of solution boiling point of pure solvent) (eq. 2) The term i is called the van' Hoff factor and it represents the effective ratio of independently moving solute particles in solution relative to the number of formula units of solute particles before dissolution. For instance, when an ionic compound such as NaCl dissolves in water it dissociates into its constituent ions Na and Cl, therefore, the theoretical ratio of independently moving solute particles in solution relative to the formula units of NaCl is 2 . In reality, however, as a reault of random motion, there will be instances when the two ions cinn momentarily recombine, reducing that ratio from its theoretical value. The real van't Hoff fictor of a solute in a solvent, therefore, will always be less than its theoretical value and must always be determined experimentally. Additionally, the real van' Hoff factor of a solute decreases as a solute's concentration increases, precisely because an increased solute concentration increases the probability of ion recombination. The term m is the molality of the solution: molality(m)=kgofsaipmemolesafsalute Molality is another way to represent a solution's concentration that is similar to its molarity. but different in that the former is a temperature-independent quantity whereas the latter is sensitive to temperature changes because volume is temperature-dependent. Along with boiling point elevation, solutions will have lower freezing points relative to the freexing point of the pure solvent: this is called freezing point depression: Tf=iKfm (cq. 4) The terms Kb and Kr are the boiling point and freezing point constants, respectively, of the solvent. These constants are independent of the solute(s) present in the solution. In this experiment the solubility of NaCl at room temperature will be determined as well as its van't Hoff factor at another concentration. Experimental Materials Experimental Procedure Part 1: Determining the solubility of NaCl in water at room temperature 1. Obtain two thermometers and record the temperature in the laboratory. Note: verify. that both thermometers are registering the same temperature reading. 2. Fill a 150mL beaker approximately halfway with distilled water, place on top of a hot plate to boil, add two boiling stones, and cover with a watch glass. Note: use a midrange setting on the hot plate to ensure a gentle boil. 3. While the distilled water is being heated, wrap parafilm or tape around the two thermometers and carefully secure them to a buret holder set on a ring stand. a. When the water appears to have reached its boiling point, partially uncover the beaker by sliding the watch glass (please use heat mitta, do not touch the hot watch glass or beaker with your bare hands) b. Carefully lower the buret holder, and insert the thermometers to measure and record the boiling point, Note: verify that both thermometers are registering the same temperature reading. 4. Perform steps 57 in two trials either in sequence or concurrently in pairs: 5. Rocord the following masses in sequence (note: set the digital scale readings to sero only once, at the beginning, before the first mass measurement): a. An empty 50mL benker and record the mass b. Place a magnetic stirring bar in the 50mL beaker and record the mass c. Add approximately 5g of NaCl to the beaker (do not remove the magnetic stirring bar) and record the mass 6. Place the 50mL beaker with magnetic stirring bar and NaCl on an unheated hot plate; turn on the stirrer (use a low-to-moderate spin setting and be careful not to accidentally turn the heating dial instead) and add distilled water in the following manner: a. Fill a 25.0mL graduated cylinder with distilled water and transfer 10.0mL of distilled water to the beaker from it with a dropper while continuously stirring b. Continue to transfer distilled water to the beaker, 1.0mL at a time, allowing 60 seconds of stirring between transfers, until all the NaCl appears to have dissolved and record the total volume transferred 7. Weigh the beaker with all its contents and determine the following: a. The mass of NaCl dissolved and record in the data sheet b. The total mass of solution in the beaker and record in the data sheet c. The mass percentage of NaCl in the solution and record in the data sheet d. Calculate the solubility of NaCl and record in the data sheet 8. Average the solubilities determined in both trials, record in the data sheet, and determine the error percentage relative to the accepted/known solubility at 25C. 9. For both trials, place the magnetic holder under the beakers to hold the magnetic: stirring bars in place and carefully pour the saturated NaCl solutions from the beakers. to 25mL graduated cylinders and record their total volumes. a. Determine the densities of the saturated NaCl solutions b. Determine the molarities of the saturated NaCl solutions c. Determine the molalities of the saturated NaCl solutions Part 2: Determining the van't Hoff factor of NaCl in water at different concentrations 10. Continue using the solutions produced in Part 1 in separate trials in Part 2 11. Using a clean dropper transfer 10.0mL of the saturated NaCl solution to a 10mL graduated cylinder, then transfer that amount to a 25mL. Erlenmeyer flask labelled gentle boil (remember to use a watch glass use a mid-range setting in the heating dial) and record the boiling point (remember also to always use the heat mitts!). 12. Using a clean dropper transfer 2.0mL of the saturated NaCl solution to a 10mL. graduated cylinder. Using a second 10mL graduated cylinder, measure 6.0mL of distilled water and then transfer that water to the first graduated cylinder. Record the volume of the mixture, then transfer it to a 25mL Erlenmeyer flask labelled "diluted NaCl " and add two boiling stones. Heat the solution on a hot plate to a gentle boil and record the boiling point. 13. Determine the following (note: ppure20=1.00g/mL and KbAHj0=0.51K/m ): a. Based on the density and mass percentages of the saturated solution and the density of pure water, calculate the molality of the diluted NaCl solution. b. Based on the density mass percentage of NaCl in the saturated solution and the total volume of the diluted mixture calculate the density and molarity of the diluted NaCl solution. c. Determine the vank Hoff factor for both NaCl solutions Data Sheet Trial 1 Trial 2 1. Room temperature and dist 2. Masses of 50mL beakers 3. Masses of 50mL beakers + stirring bars 4. Masses of 50mL beakers + stirring bars +NaCl 5. Total volumes of water transferred to 50mL beakers 6. Masses of 50mL beakers 7. Masses of NaCl dissolved 8. Total masses of saturated NaCl solutions 9. Total volumes of saturated NaCl solutions 10. Mass percentages of NaCl in solutions 11. Solubility of saturated NaCl solution (experimental) 12. Average solubilities of saturated NaCl solutions 13. Solubility of saturated Nach 14. Solubility Percent error 15. Densities of saturated NaCl solutions 16. Molarities of saturated NaCl solutions: 17. Molalities of saturated NaCl solutions 18. Boiling points of saturated NaCl solutions 19. Boiling points of dituted NaCl solutions 20. Volumes of diluted NaCl solutione 21. Molslitics of diluted NaCl solutions (refer to step 13) solubility =100mLofsolventmassofsolute(g) or mLofsolventmassofsolute(mg) or 1Lofsolutionmolesofsolute(M) Certain physical properties of liquid-phased solutions differ in specifie ways from those of the pure solvent and these differences depend only on how much solute is present. Properties of this type are called colligative properties and they include, but are not limited to, changes to boiling and freezing points, changes to vapor pressure, and changes to osmotic pressure. Boiling points of solutions, for instance, are higher than those of the pure solvent. This is called boiling point elevation and the change in boiling point can be determined using the following equation: Tb=iKbm In this equation Tb is the difference in boiling point of the solution relative to that of the pure solvent. Tb=( (bolling point of solution boiling point of pure solvent) (eq. 2) The term i is called the van' Hoff factor and it represents the effective ratio of independently moving solute particles in solution relative to the number of formula units of solute particles before dissolution. For instance, when an ionic compound such as NaCl dissolves in water it dissociates into its constituent ions Na and Cl, therefore, the theoretical ratio of independently moving solute particles in solution relative to the formula units of NaCl is 2 . In reality, however, as a reault of random motion, there will be instances when the two ions cinn momentarily recombine, reducing that ratio from its theoretical value. The real van't Hoff fictor of a solute in a solvent, therefore, will always be less than its theoretical value and must always be determined experimentally. Additionally, the real van' Hoff factor of a solute decreases as a solute's concentration increases, precisely because an increased solute concentration increases the probability of ion recombination. The term m is the molality of the solution: molality(m)=kgofsaipmemolesafsalute Molality is another way to represent a solution's concentration that is similar to its molarity. but different in that the former is a temperature-independent quantity whereas the latter is sensitive to temperature changes because volume is temperature-dependent. Along with boiling point elevation, solutions will have lower freezing points relative to the freexing point of the pure solvent: this is called freezing point depression: Tf=iKfm (cq. 4) The terms Kb and Kr are the boiling point and freezing point constants, respectively, of the solvent. These constants are independent of the solute(s) present in the solution. In this experiment the solubility of NaCl at room temperature will be determined as well as its van't Hoff factor at another concentration. Experimental Materials Experimental Procedure Part 1: Determining the solubility of NaCl in water at room temperature 1. Obtain two thermometers and record the temperature in the laboratory. Note: verify. that both thermometers are registering the same temperature reading. 2. Fill a 150mL beaker approximately halfway with distilled water, place on top of a hot plate to boil, add two boiling stones, and cover with a watch glass. Note: use a midrange setting on the hot plate to ensure a gentle boil. 3. While the distilled water is being heated, wrap parafilm or tape around the two thermometers and carefully secure them to a buret holder set on a ring stand. a. When the water appears to have reached its boiling point, partially uncover the beaker by sliding the watch glass (please use heat mitta, do not touch the hot watch glass or beaker with your bare hands) b. Carefully lower the buret holder, and insert the thermometers to measure and record the boiling point, Note: verify that both thermometers are registering the same temperature reading. 4. Perform steps 57 in two trials either in sequence or concurrently in pairs: 5. Rocord the following masses in sequence (note: set the digital scale readings to sero only once, at the beginning, before the first mass measurement): a. An empty 50mL benker and record the mass b. Place a magnetic stirring bar in the 50mL beaker and record the mass c. Add approximately 5g of NaCl to the beaker (do not remove the magnetic stirring bar) and record the mass 6. Place the 50mL beaker with magnetic stirring bar and NaCl on an unheated hot plate; turn on the stirrer (use a low-to-moderate spin setting and be careful not to accidentally turn the heating dial instead) and add distilled water in the following manner: a. Fill a 25.0mL graduated cylinder with distilled water and transfer 10.0mL of distilled water to the beaker from it with a dropper while continuously stirring b. Continue to transfer distilled water to the beaker, 1.0mL at a time, allowing 60 seconds of stirring between transfers, until all the NaCl appears to have dissolved and record the total volume transferred 7. Weigh the beaker with all its contents and determine the following: a. The mass of NaCl dissolved and record in the data sheet b. The total mass of solution in the beaker and record in the data sheet c. The mass percentage of NaCl in the solution and record in the data sheet d. Calculate the solubility of NaCl and record in the data sheet 8. Average the solubilities determined in both trials, record in the data sheet, and determine the error percentage relative to the accepted/known solubility at 25C. 9. For both trials, place the magnetic holder under the beakers to hold the magnetic: stirring bars in place and carefully pour the saturated NaCl solutions from the beakers. to 25mL graduated cylinders and record their total volumes. a. Determine the densities of the saturated NaCl solutions b. Determine the molarities of the saturated NaCl solutions c. Determine the molalities of the saturated NaCl solutions Part 2: Determining the van't Hoff factor of NaCl in water at different concentrations 10. Continue using the solutions produced in Part 1 in separate trials in Part 2 11. Using a clean dropper transfer 10.0mL of the saturated NaCl solution to a 10mL graduated cylinder, then transfer that amount to a 25mL. Erlenmeyer flask labelled gentle boil (remember to use a watch glass use a mid-range setting in the heating dial) and record the boiling point (remember also to always use the heat mitts!). 12. Using a clean dropper transfer 2.0mL of the saturated NaCl solution to a 10mL. graduated cylinder. Using a second 10mL graduated cylinder, measure 6.0mL of distilled water and then transfer that water to the first graduated cylinder. Record the volume of the mixture, then transfer it to a 25mL Erlenmeyer flask labelled "diluted NaCl " and add two boiling stones. Heat the solution on a hot plate to a gentle boil and record the boiling point. 13. Determine the following (note: ppure20=1.00g/mL and KbAHj0=0.51K/m ): a. Based on the density and mass percentages of the saturated solution and the density of pure water, calculate the molality of the diluted NaCl solution. b. Based on the density mass percentage of NaCl in the saturated solution and the total volume of the diluted mixture calculate the density and molarity of the diluted NaCl solution. c. Determine the vank Hoff factor for both NaCl solutions Data Sheet Trial 1 Trial 2 1. Room temperature and dist 2. Masses of 50mL beakers 3. Masses of 50mL beakers + stirring bars 4. Masses of 50mL beakers + stirring bars +NaCl 5. Total volumes of water transferred to 50mL beakers 6. Masses of 50mL beakers 7. Masses of NaCl dissolved 8. Total masses of saturated NaCl solutions 9. Total volumes of saturated NaCl solutions 10. Mass percentages of NaCl in solutions 11. Solubility of saturated NaCl solution (experimental) 12. Average solubilities of saturated NaCl solutions 13. Solubility of saturated Nach 14. Solubility Percent error 15. Densities of saturated NaCl solutions 16. Molarities of saturated NaCl solutions: 17. Molalities of saturated NaCl solutions 18. Boiling points of saturated NaCl solutions 19. Boiling points of dituted NaCl solutions 20. Volumes of diluted NaCl solutione 21. Molslitics of diluted NaCl solutions (refer to step 13)