Answered step by step
Verified Expert Solution
Question
1 Approved Answer
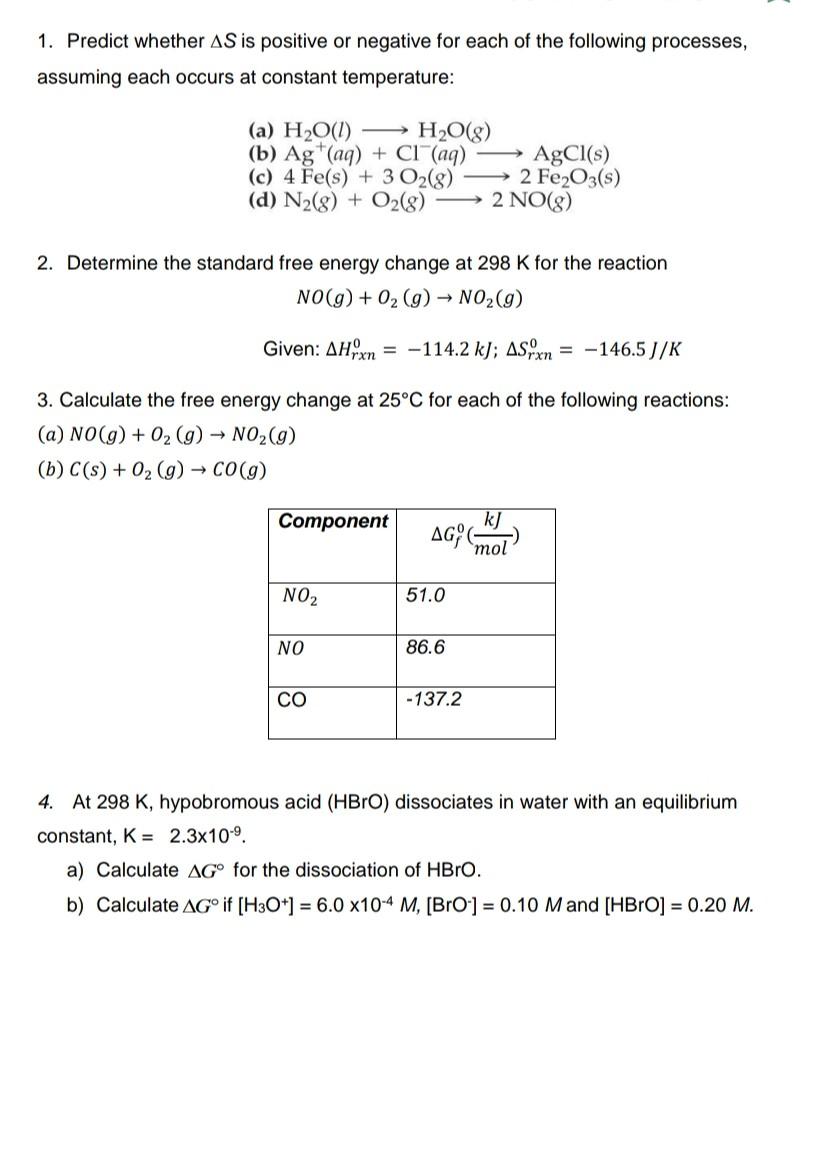
Solution 1. Predict whether S is positive or negative for each of the following processes, assuming each occurs at constant temperature: (a) H2O(l)H2O(g) (b) Ag+(aq)+Cl(aq)AgCl(s)
Solution
1. Predict whether S is positive or negative for each of the following processes, assuming each occurs at constant temperature: (a) H2O(l)H2O(g) (b) Ag+(aq)+Cl(aq)AgCl(s) (c) 4Fe(s)+3O2(g)2Fe2O3(s) (d) N2(g)+O2(g)2NO(g) 2. Determine the standard free energy change at 298K for the reaction NO(g)+O2(g)NO2(g) Given: Hrxn0=114.2kJ;Srxn0=146.5J/K 3. Calculate the free energy change at 25C for each of the following reactions: (a) NO(g)+O2(g)NO2(g) (b) C(s)+O2(g)CO(g) 4. At 298K, hypobromous acid (HBrO) dissociates in water with an equilibrium constant, K=2.3109. a) Calculate G for the dissociation of HBrO. b) Calculate G if [H3O+]=6.0104M, [BrO]=0.10M and [HBrO]=0.20MStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


