Question: (a) Carefully complete the energy diagram by drawing a curve that accurately shows the progress of the reaction, beginning at the reactants, moving through
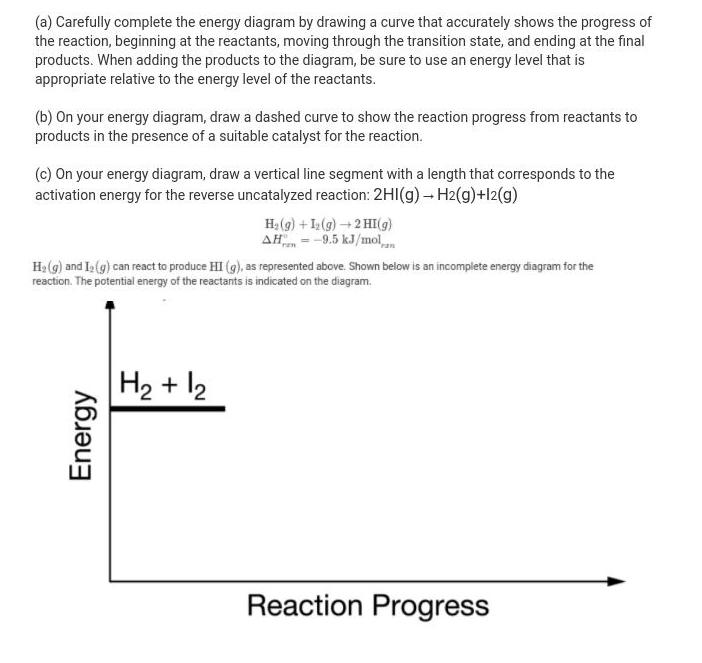
(a) Carefully complete the energy diagram by drawing a curve that accurately shows the progress of the reaction, beginning at the reactants, moving through the transition state, and ending at the final products. When adding the products to the diagram, be sure to use an energy level that is appropriate relative to the energy level of the reactants. (b) On your energy diagram, draw a dashed curve to show the reaction progress from reactants to products in the presence of a suitable catalyst for the reaction. (c) On your energy diagram, draw a vertical line segment with a length that corresponds to the activation energy for the reverse uncatalyzed reaction: 2HI(g) - H2(g)+l2(g) H, (g) + (g)+2 HI(g) AH = -9.5 kJ/mol H (g) and I(g) can react to produce HI (g), as represented above. Shown below is an incomplete energy diagram for the reaction. The potential energy of the reactants is indicated on the diagram. H2 + 2 Reaction Progress Energy
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it
Since the reaction is exothermic the energy of products will be lower than the energy of ... View full answer

Get step-by-step solutions from verified subject matter experts


