Solve appropriately
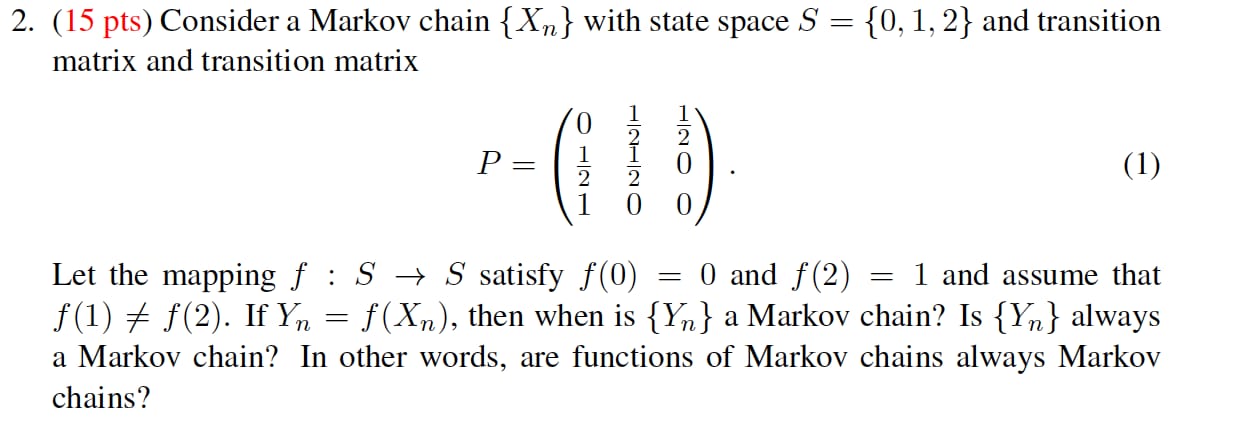
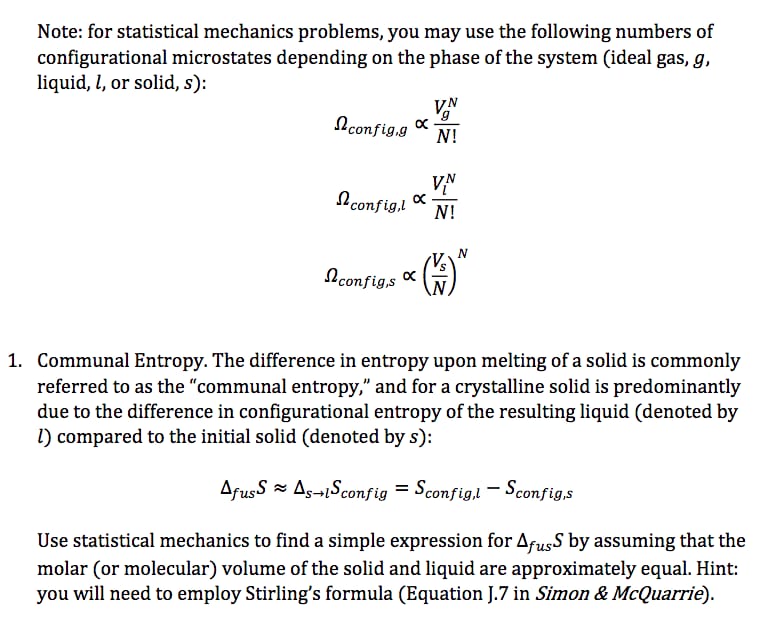
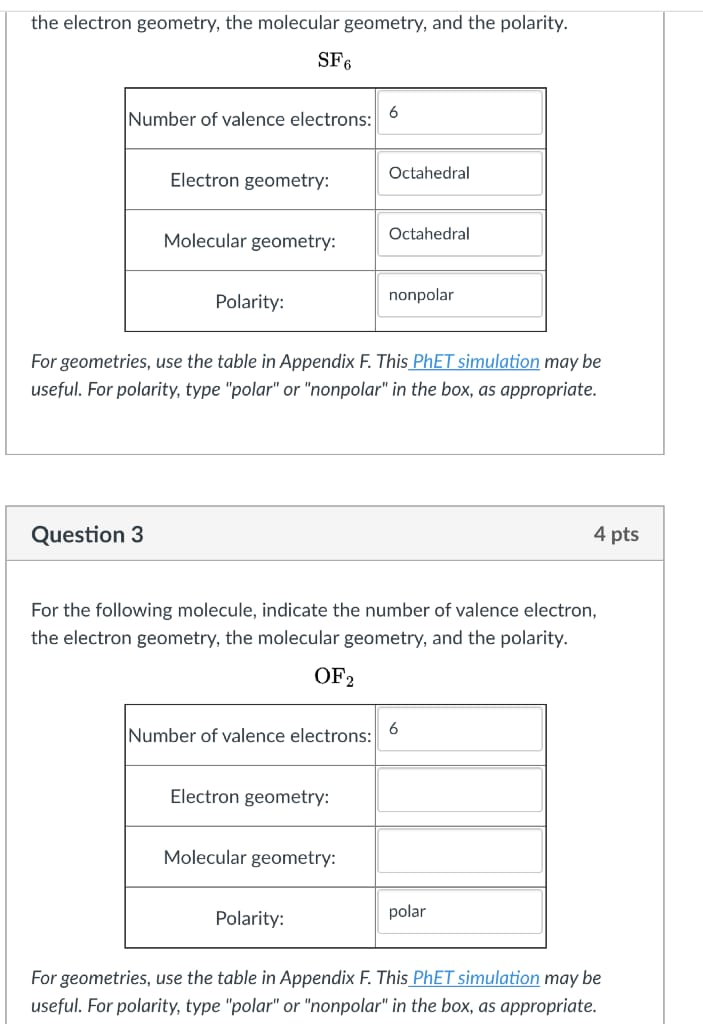
2. (15 pts) Consider a Markov chain { Xn } with state space S = {0, 1, 2} and transition matrix and transition matrix P = O ON/H HN/H O (1) Let the mapping f : S - S satisfy f(0) = 0 and f(2) = 1 and assume that f(1) # f(2). If Yn = f(Xn), then when is { Yn } a Markov chain? Is {Yn } always a Markov chain? In other words, are functions of Markov chains always Markov chains?For a 4-unit class like Statistics, students should spend average of 12 hours studying for the class. A survey was done on 22 students, and the distribution of total study hours per week is bell-shaped with a mean of 14 hours and a standard deviation of 3.2 hours. Use the Empirical Rule to answer the following questions. a) 68% of the students spend between hours and hours on Statistics each week. b) 95% of the students spend between hours and hours on Statistics each week. c) 99.7% of the students spend between hours and hours on Statistics each week.Note: for statistical mechanics problems, you may use the following numbers of congurations] microstates depending on the phase of the system (ideal gas, 9, liquid, 1, or solid, 5): 1. Communal Entropy. The difference in entropy upon melting of a solid is commonly referred to as the "communal entropy," and for a crystalline solid is predominantly due to the difference in congurational entropy of the resulting liquid (denoted by I) compared to the initial solid (denoted by s): rms 2' sdlsconfig = configJ Sconfig Use statistical mechanics to find a simple expression for drugs by assuming that the molar (or molecular) volume of the solid and liquid are approximately equal. Hint: you will need to employ Stirling's formula (Equation 1.? in Simon 9 McQuarrfe). the electron geometry, the molecular geometry, and the polarity. SF : For geometries. use the table in Appendix F. This PhET simulation may be useful. For polarity, type "polar" or \"nonpoiar\" in the box, as appropriate. Question 3 For the following molecule. indicate the number of yalence electron, the electron geometry, the molecular geometry, and the polarity. OFE : ; ; For geometries, use the table in Appendix F. This PhET simulation may be useful. Far polarity, type "polar" or \"nonpoiar\" in the box, as appropriate