Answered step by step
Verified Expert Solution
Question
1 Approved Answer
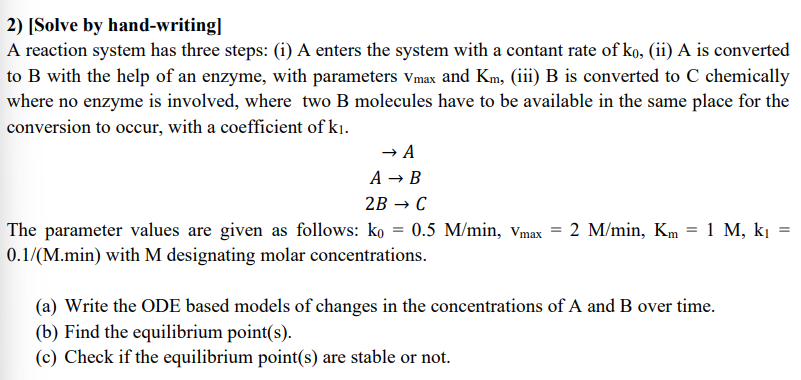
[ Solve by hand - writing ] A reaction system has three steps: ( i ) A enters the system with a contant rate of
Solve by handwriting
A reaction system has three steps: i A enters the system with a contant rate of ii is converted to with the help of an enzyme, with parameters and iii B is converted to chemically where no enzyme is involved, where two B molecules have to be available in the same place for the conversion to occur, with a coefficient of
The parameter values are given as follows: with designating molar concentrations.
a Write the ODE based models of changes in the concentrations of A and B over time.
b Find the equilibrium points
c Check if the equilibrium points are stable or not.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


