Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve Calculated Data and Questions 1-4 (show calculations. data given. Antacid tablets are sold over the counter to treat heartburn and indigestion due to excess
solve "Calculated Data" and Questions 1-4 (show calculations. data given. 


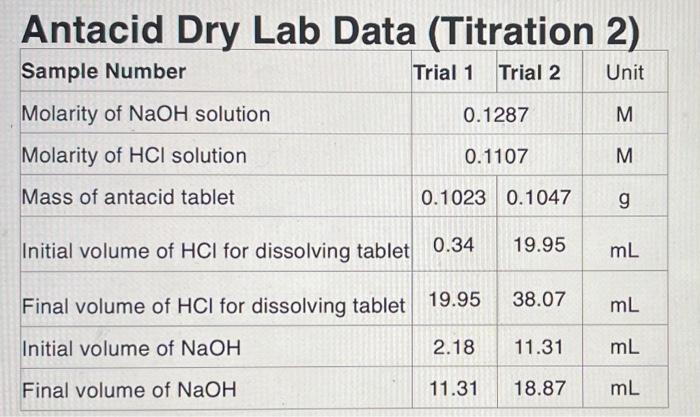
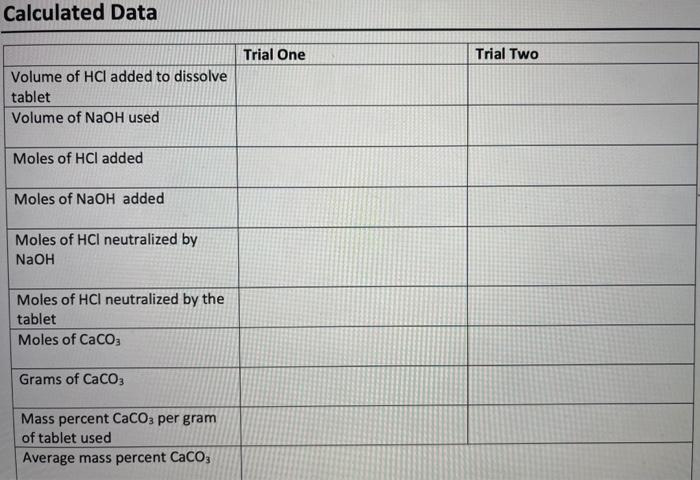


Antacid tablets are sold over the counter to treat heartburn and indigestion due to excess stomach acid. Antacid tablets commonly contain binders which hold the tablet together, flavoring agents, and most importantly one or more active ingredients such as calcium carbonate (CaCO3), magnesium hydroxide (Mg(OH)2), and sodium bicarbonate (NaHCO3) which neutralize the excess stomach acid. Tums antacid tablets contain calcium carbonate and neutralize hydrochloric acid by reaction 1. Reaction 1 2HCl(aq) + CaCO3(s) CaCl2(aq) + CO2(g) + H2O *As can be seen from the balanced chemical reaction, 2 moles of HCl react with 1 mole of CaCO3. Similarly, Rolaids tablets contain magnesium hydroxide in addition to calcium carbonate. The neutralization reaction between magnesium hydroxide and hydrochloric acid is described in reaction 2. Reaction 2 2HCl(aq) + Mg(OH)2(5) MgCl2(aq) + H2O) The antacid tablets that will be used in this experiment contain calcium carbonate as the active ingredient. Due to variations in the active ingredients, and limited solubility of some of the binding agents, a back-titration will be used in this experiment. The goal is to add an excess amount of standardized hydrochloric acid to completely neutralize the antacid tablet. Next, a standardized solution of sodium hydroxide (NaOH) is added to neutralize the excess acid (Reaction 3). By knowing the amount of HCl reacted with the NaOH, the portion of HCl that reacted with the tablet can be calculated and related back to original mass of antacid tablet used. Reaction 3 HCl(aq)(excess) + NaOH(aq) NaCl(aq) + H20W Consider the following example calculation where 0.7496 grams of an antacid is neutralized Consider the following example calculation where 0.7496 grams of an antacid is neutralized with 25.00 mL of 0.5900 M HCl. Then, 4.82 mL of 1.064 M NaOH is used for the back-titration. The number of moles of HCl added is calculated from the volume of HCl and its molarity. Equation 1 12 25.00 mL x X 1000 mL 0.5900 mol 11 0.01475 mol HCL 1L Similarly, the number of moles of NaOH added in the back-titration is found to be 0.00513 moles NaOH. 1.064 mol Equation 2 4.82 mL x x 0.00513 mol NaOH 1000 ml 1L Since NaOH and HCl react in a 1:1 ratio, the excess number of moles of HCI must also be 0.00513 moles. 1 mol HCL Equation 3 0.00513 mol NaOH x 0.00513 mol HCl 1 mol NaOH Subtracting this number of moles of HCl from the amount originally added gives the number of moles neutralized by the antacid tablet. Equation 4 0.01475 mol HCL - 0.00513 mol HCl = 0.00962 mol HCl neutralized Recall from Reaction 1, the stoichiometric ration between hydrochloric acid and calcium carbonate is 2:1 and, therefore, the number of moles and mass of calcium carbonate can be calculated from its molar mass (equations 5 and 6). = Equation 5 1 mol CaCO3 2 mol HCL 0.00962 mol HCLX 0.00481 mol CaCO3 = Equation 6 100.09 gram CaCO3 1 mol CaCO3 0.00481 mol CaCO3 x = 0.481 grams CaCO3 The percent calcium carbonate in the antacid tablet can then be calculated from the starting mass Equation 7 0.481 grams Caco, x 100% = 64.2% CaCO3 0.7496 grams When performing this experiment, it is important that the antacid tablet-HCl mixture is heated Antacid Dry Lab Data (Titration 2) Sample Number Trial 1 Trial 2 Unit 0.1287 M Molarity of NaOH solution Molarity of HCl solution 0.1107 M Mass of antacid tablet 0.1023 0.1047 g Initial volume of HCl for dissolving tablet 0.34 19.95 mL Final volume of HCl for dissolving tablet 19.95 38.07 mL Initial volume of NaOH 2.18 11.31 mL Final volume of NaOH 11.31 18.87 mL Calculated Data Trial One Trial Two Volume of HCI added to dissolve tablet Volume of NaOH used Moles of HCl added Moles of NaOH added Moles of HCl neutralized by NaOH Moles of HCl neutralized by the tablet Moles of CaCO3 Grams of CaCO3 Mass percent CaCO3 per gram of tablet used Average mass percent CaCO3 1. Why was it necessary to perform a back-titration to determine the efficiency of an antacid instead of just titrating the tablet directly with HCI? 2. A student performs this experiment but forgets to remove air bubbles from the NaOH buret valve. Would the measured volume of NaOH added in the back-titration be incorrectly high or low? How would this error affect the results of the experiment? 3. The stoichiometry in this reaction was 2 mole of acid to 1 mole of calcium carbonate or 1 mole of magnesium hydroxide. How you the amount of NaOH needed for the back- titration be affected if the antacid tablet contained sodium bicarbonate (NaHCO3)? 4. A student was performing this experiment with an antacid tablet which contained CaCO3 but forgot to heat the antacid solution prior to starting the titration. As sodium hydroxide was added the student noticed the formation of an insoluble white precipitate. a. Identify the precipitate. (Hint: the precipitate was soluble under acidic conditions) b. Would the presence of this precipitate cause the students experimentally determined volume of NaOH, required for the back-titration, to be incorrectly high or incorrectly low? Briefly explain. C. Would the resulting calculated number of moles of HCl, neutralized by the antacid tablet, be incorrectly large or small? Briefly explain 






Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


