Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve for parts b and c. Hinge: the solutions should match with answers provided 2. Liquid methyl ethyl ketone (MEK) is introduced into a chamber
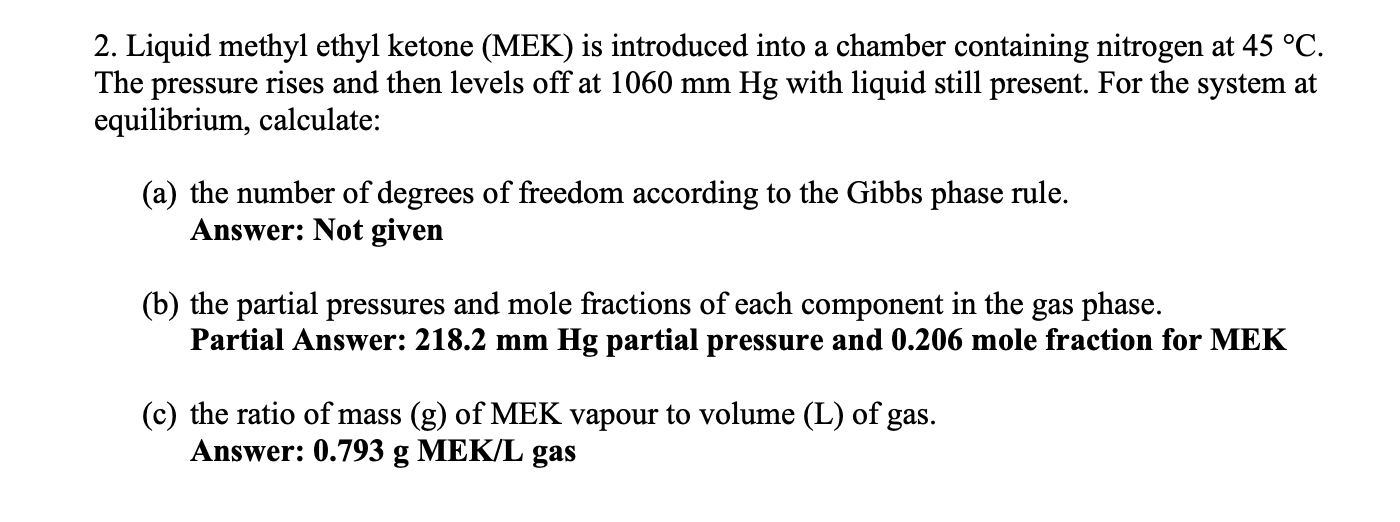 Solve for parts b and c.
Solve for parts b and c.
Hinge: the solutions should match with answers provided
2. Liquid methyl ethyl ketone (MEK) is introduced into a chamber containing nitrogen at 45 C. The pressure rises and then levels off at 1060 mm Hg with liquid still present. For the system at equilibrium, calculate: (a) the number of degrees of freedom according to the Gibbs phase rule. Answer: Not given (b) the partial pressures and mole fractions of each component in the gas phase. Partial Answer: 218.2 mm Hg partial pressure and 0.206 mole fraction for MEK (c) the ratio of mass (g) of MEK vapour to volume (L) of gas. Answer: 0.793 g MEK/L gasStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


