Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve for the unknowns as well please. I would like to see the process and compare with mine C6H5CH3+H2C6H6+CH4 In the process shown below 109
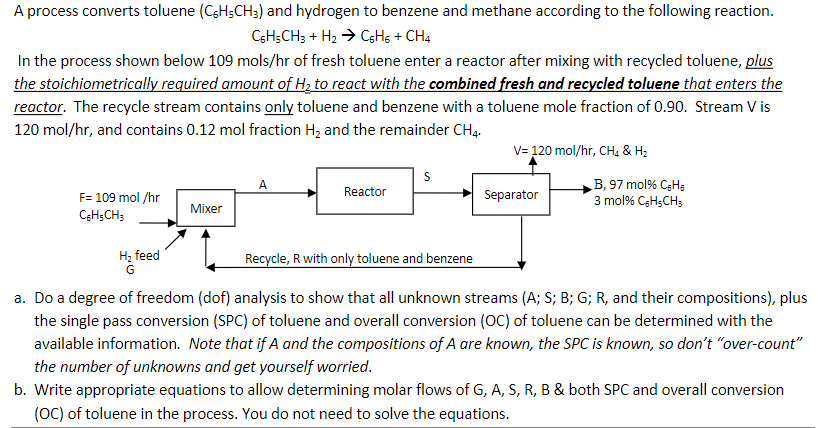
Solve for the unknowns as well please. I would like to see the process and compare with mine
C6H5CH3+H2C6H6+CH4 In the process shown below 109 mols/hr of fresh toluene enter a reactor after mixing with recycled toluene, plus the stoichiometrically required amount of H2 to react with the combined fresh and recycled toluene that enters the reactor. The recycle stream contains only toluene and benzene with a toluene mole fraction of 0.90. Stream V is 120mol/hr, and contains 0.12mol fraction H2 and the remainder CH4. a. Do a degree of freedom (dof) analysis to show that all unknown streams ( A;S;B;G;R, and their compositions), plus the single pass conversion (SPC) of toluene and overall conversion (OC) of toluene can be determined with the available information. Note that if A and the compositions of A are known, the SPC is known, so don't "over-count" the number of unknowns and get yourself worried. b. Write appropriate equations to allow determining molar flows of G,A,S,R,B \& both SPC and overall conversion (OC) of toluene in the process. You do not need to solve the equationsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


