Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve it in matlab. For concerning the Gas-Phase Reaction in a Microreactor-Molar Flow Rates. The reaction in the gas phase is given below. 2NOCl2NO+Cl2 is
solve it in matlab. 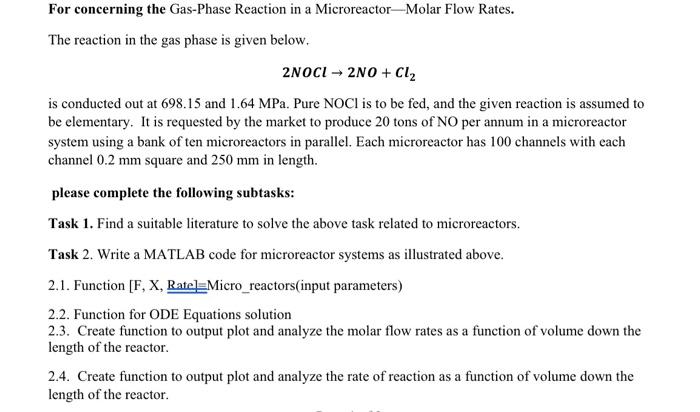

For concerning the Gas-Phase Reaction in a Microreactor-Molar Flow Rates. The reaction in the gas phase is given below. 2NOCl2NO+Cl2 is conducted out at 698.15 and 1.64MPa. Pure NOCl is to be fed, and the given reaction is assumed to be elementary. It is requested by the market to produce 20 tons of NO per annum in a microreactor system using a bank of ten microreactors in parallel. Each microreactor has 100 channels with each channel 0.2mm square and 250mm in length. please complete the following subtask: Task 1. Find a suitable literature to solve the above task related to microreactors. Task 2. Write a MATLAB code for microreactor systems as illustrated above. 2.1. Function \( [\mathrm{F}, \mathrm{X}, \underline{\text { Rate }] \equiv \text { Micro_reactors(input parameters) }} \) 2.2. Function for ODE Equations solution 2.3. Create function to output plot and analyze the molar flow rates as a function of volume down the length of the reactor. 2.4. Create function to output plot and analyze the rate of reaction as a function of volume down the length of the reactor. 2.5. Create function to output plot and analyze the conversion as a function of volume down the length of the reactor. Information To produce 20 tons per year of NO at 80% conversion would require a feed rate of 0.02mol/s of NOCl, or 2.26105mol/s per channel. The rate constant is (k=0.29 with activation energy 24 Kcal.mol1 at 500K ), volume of each channel is 105dm3 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


