Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve it with overall effectiveness factor. Solve it with Internal Diffusion Limitation. Explain Solve it with External Diffusion Limitation (External Mass Transfer Limitation). Explain Example
Solve it with overall effectiveness factor.
Solve it with Internal Diffusion Limitation. Explain
Solve it with External Diffusion Limitation (External Mass Transfer Limitation). Explain
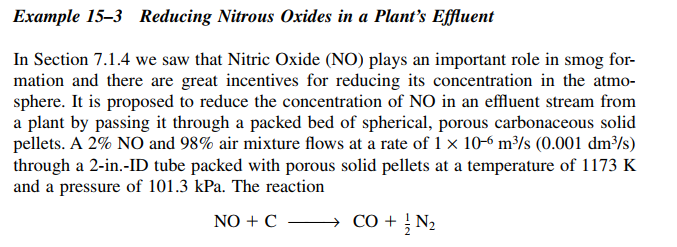
Example 15-3 Reducing Nitrous Oxides in a Plant's Effluent In Section 7.1.4 we saw that Nitric Oxide (NO) plays an important role in smog formation and there are great incentives for reducing its concentration in the atmosphere. It is proposed to reduce the concentration of NO in an effluent stream from a plant by passing it through a packed bed of spherical, porous carbonaceous solid pellets. A 2%NO and 98% air mixture flows at a rate of 1106m3/s(0.001dm3/s) through a 2-in.-ID tube packed with porous solid pellets at a temperature of 1173K and a pressure of 101.3kPa. The reaction NO+CCO+21N2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


