Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve part a and part b please The half - life for the radioactive decay of U - 2 3 8 is 4 . 5
Solve part a and part b please
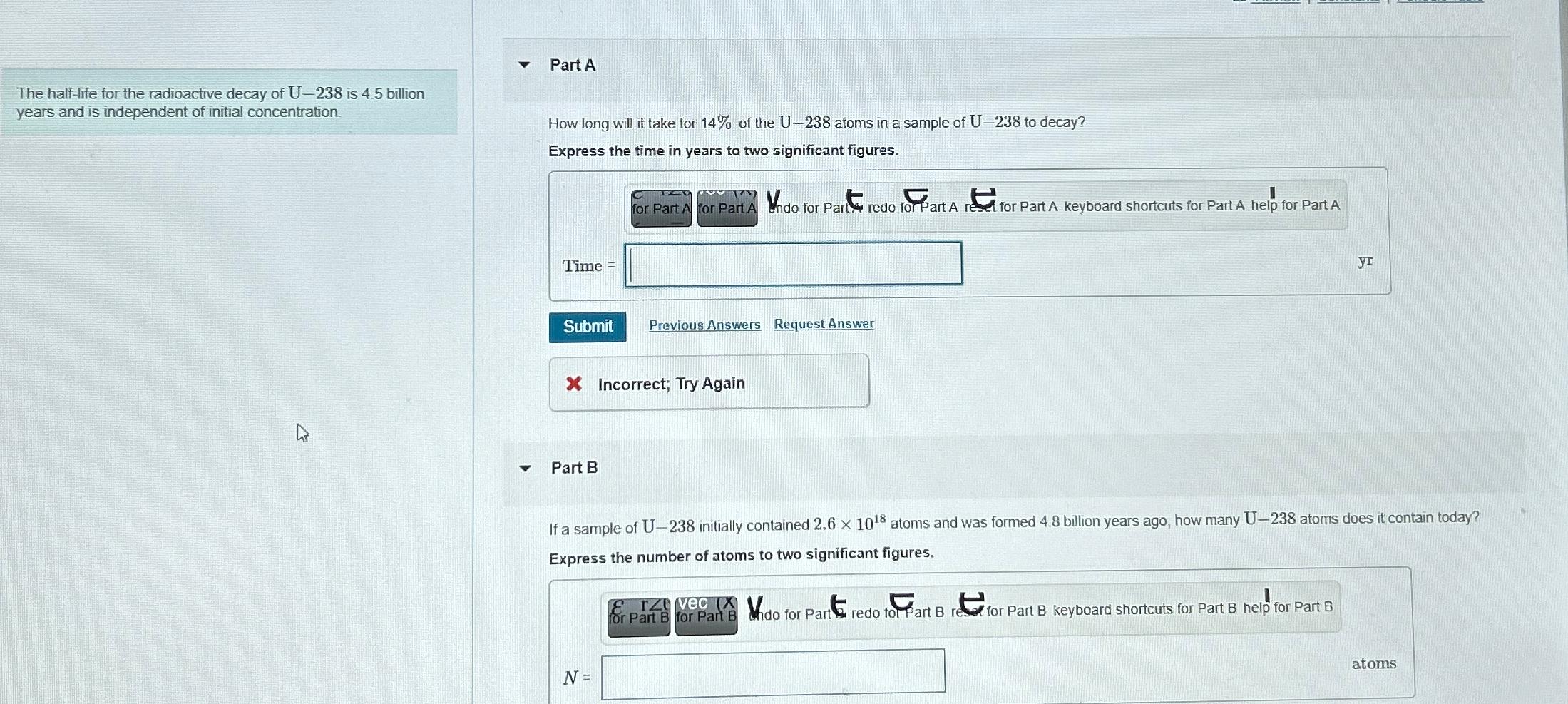
The halflife for the radioactive decay of U is billion years and is independent of initial concentration.
Part A
How long will it take for of the atoms in a sample of to decay?
Express the time in years to two significant figures.
Time
Previous Answers Request Answer
Incorrect; Try Again
Part B
If a sample of initially contained atoms and was formed billion years ago, how many atoms does it contain today? Express the number of atoms to two significant figures.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


