Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve question 1 and 2 1.1. Use the ideal gas equation of state to estimate the molar volume in m3/mol and the density of air
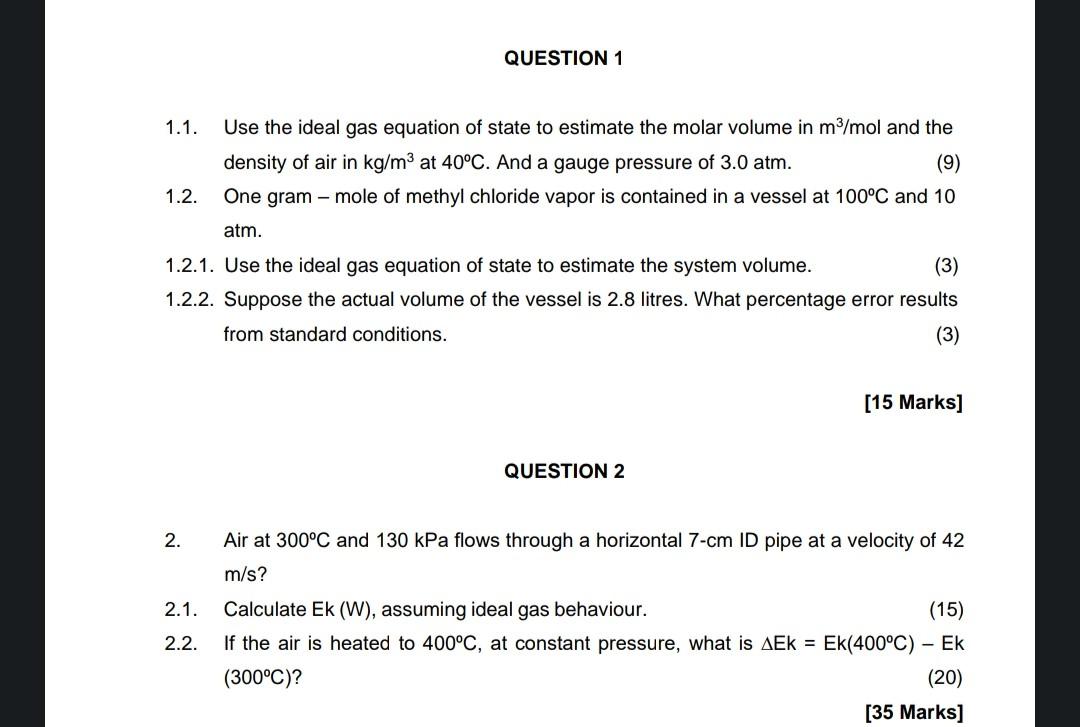
solve question 1 and 2
1.1. Use the ideal gas equation of state to estimate the molar volume in m3/mol and the density of air in kg/m3 at 40C. And a gauge pressure of 3.0atm. (9) 1.2. One gram - mole of methyl chloride vapor is contained in a vessel at 100C and 10 atm. 1.2.1. Use the ideal gas equation of state to estimate the system volume. 1.2.2. Suppose the actual volume of the vessel is 2.8 litres. What percentage error results from standard conditions. (3) [15 Marks] QUESTION 2 2. Air at 300C and 130kPa flows through a horizontal 7-cm ID pipe at a velocity of 42 m/s? 2.1. Calculate Ek(W), assuming ideal gas behaviour. 2.2. If the air is heated to 400C, at constant pressure, what is Ek=Ek(400C)Ek (300C)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


