Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve question 1 and 2 Post Lab Questions 1. Is there a significant difference in the results between the samples? 2. What will happen to
solve question 1 and 2 
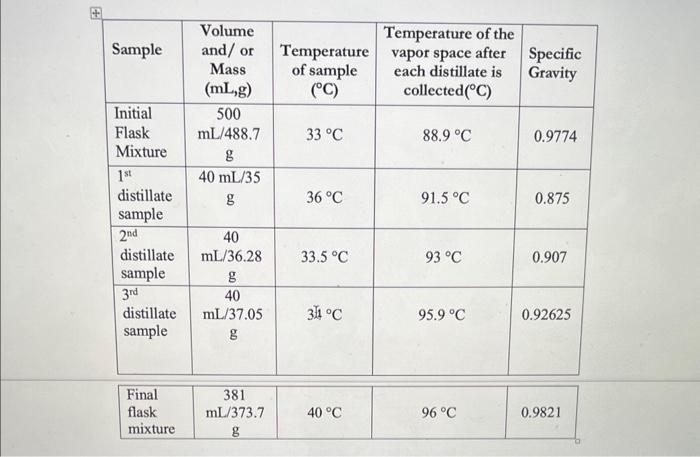
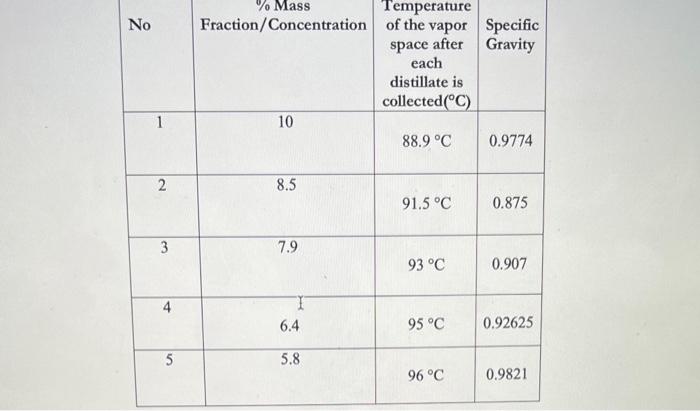
Post Lab Questions 1. Is there a significant difference in the results between the samples? 2. What will happen to the distillate if the concentration of ethanol is increased from 10% ? Explain. \begin{tabular}{|l|c|c|c|c|} \hline Sample & Volumeand/orMass(mL,g) & Temperatureofsample(C) & Temperatureofthevaporspaceaftereachdistillateiscollected(C) & SpecificGravity \\ \hline InitialFlaskMixture & 500mL/488.7g & 33C & 88.9C & 0.9774 \\ \hline 1stdistillatesample & 40mL/35g & 36C & 91.5C & 0.875 \\ \hline 2nddistillatesample & 40mL/36.28g & 33.5C & 93C & 0.907 \\ \hline 3rddistillatesample & 40mL/37.05g & 341C & 95.9C & 0.92625 \\ \hline \end{tabular} \begin{tabular}{|c|c|c|c|} \hline No & Fraction/Concentration & Temperatureofthevaporspaceaftereach & SpecificGravitydistillateiscollectedC) \\ \hline 1 & 10 & 88.9C & 0.9774 \\ \hline 2 & 8.5 & 91.5C & 0.875 \\ \hline 3 & 7.9 & 93C & 0.907 \\ \hline 4 & 6.4 & 955C & 0.92625 \\ \hline 5 & 5.8 & 96C & 0.9821 \\ \hline \end{tabular} 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


