Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve quickly for a thumbs up Question 4 5 pi The SRK EOS can be defined as: PRT Po v(O+B) b -b Where: = 0.42747(RT)
Solve quickly for a thumbs up 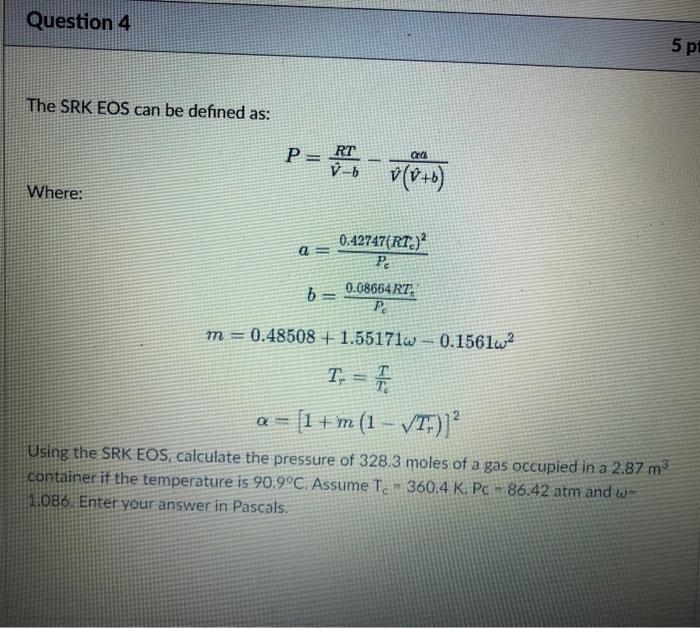
Question 4 5 pi The SRK EOS can be defined as: PRT Po v(O+B) b -b Where: = 0.42747(RT) Pc 6 = 0.08654 RT: PO m = 0.48508 + 1.55171w 0.1561w2 T. = F = [1 +'m (1 VT;)] Using the SRK EOS, calculate the pressure of 328.3 moles of a gas occupied in a 2.87 m container if the temperature is 90.9C. Assume Te - 360.4 K. Pc - 86.42 atm and we 1.086 Enter your answer in Pascals. m 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


