Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve the following question based on two trials. molarity of KMNo4 is 0.010 M and we need to solve the following question based on the
solve the following question based on two trials. 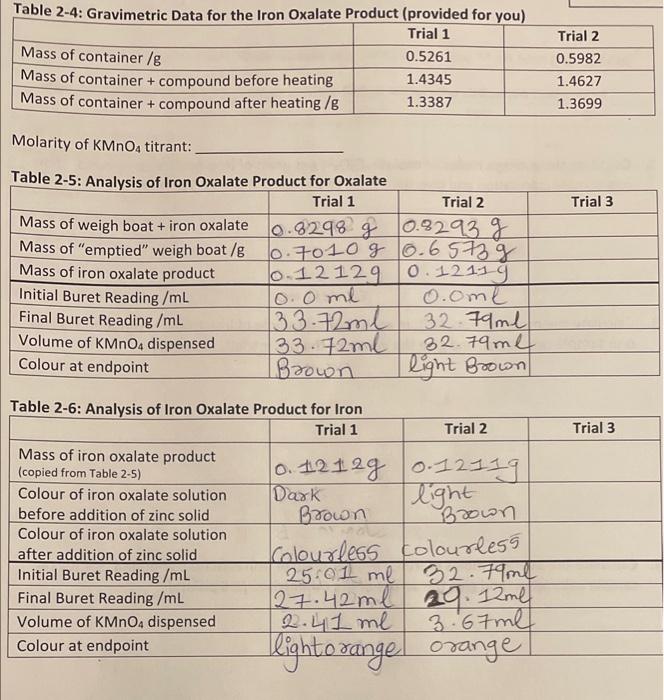


molarity of KMNo4 is 0.010 M and we need to solve the following question based on the two trials done. third trial is optional.
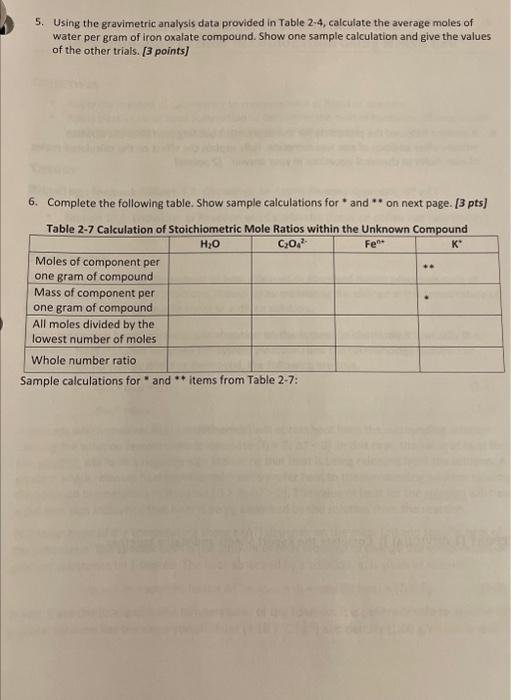
Molarity of KMnO4 titrant: Tahla o e. A...... 1. Calculate the moles of oxalate, C2O42, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [2 points] 2. Calculate the average moles of oxalate, C2O42, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 3. Calculate the moles of iron, Fen+, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [2 points] 4. Calculate the average moles of iron, Fen+, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 5. Using the gravimetric analysis data provided in Table 24, calculate the average moles of water per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [ 3 points] 6. Complete the following table. Show sample calculations for * and " on next page. [3 pts] Sample calculations for " and items trom rabie 21 : Molarity of KMnO4 titrant: Tahla o e. A...... 1. Calculate the moles of oxalate, C2O42, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [2 points] 2. Calculate the average moles of oxalate, C2O42, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 3. Calculate the moles of iron, Fen+, in each titrated sample of iron oxalate compound. Show one sample calculation and give the values of the other trials. [2 points] 4. Calculate the average moles of iron, Fen+, per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [1.5 points] 5. Using the gravimetric analysis data provided in Table 24, calculate the average moles of water per gram of iron oxalate compound. Show one sample calculation and give the values of the other trials. [ 3 points] 6. Complete the following table. Show sample calculations for * and " on next page. [3 pts] Sample calculations for " and items trom rabie 21 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


