Question
Solve the problem on the previous page (reversible reaction in a non-isothermal PFR at different feed inlet temperatures): 1- From the plots given, estimate the
Solve the problem on the previous page (reversible reaction in a non-isothermal PFR at different feed inlet temperatures): 1- From the plots given, estimate the heat of the reaction, reaction order, activation energy, heat capacities, and weight of the catalyst. If you need to make assumptions, explain, and justify them! 2- Explain and justify, clearly, how you estimated those parameters from the plots. 3- List all the equations you will use to calculate the exit conversion and describe how you will solve them. 4- Solve the equations numerically at different inlet temperatures. 5- Plot exit conversion vs. inlet temperature. 6- Plot exit conversion vs. W for 3 different temperatures, optimum T (Topt ), T > Topt, and T< Topt 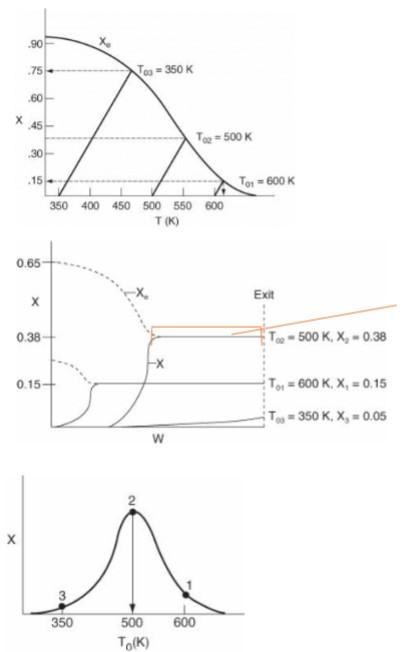
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


