Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve the report Experiment 1 Chemical Reagent Preparation Objective Prepare the chemical solutions required for the reaction rate testing including a calibration curve for the
solve the report 

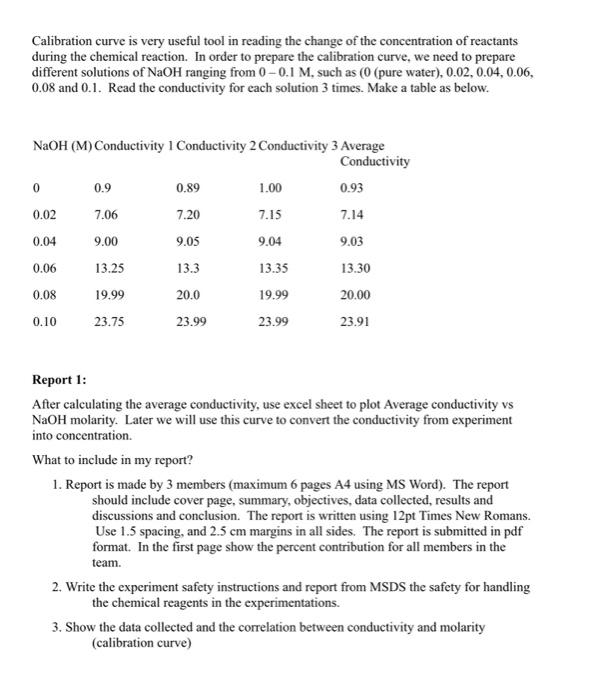
Experiment 1 Chemical Reagent Preparation Objective Prepare the chemical solutions required for the reaction rate testing including a calibration curve for the NaOH reagent. What is MSDS? Before start preparing the chemical reagents that will be used in the experiment, we need to obtain the material safety data sheet (MSDS) for all reagents in the experiment. A material safety data sheet is a technical document which provides detailed and comprehensive information on a controlled product related to - health effects of exposure to the product - hazard evaluation related to the product's handling, storage or use - measure to protect workers at risk of exposure - emergency procedures The data sheet may be written, printed or otherwise expressed and must meet the availability, design and content requirements of Workplace Hazardous Materials Information System (WHMIS) legislation. The legislation provides for flexibility of design and wording but requires that a minimum number of categories of information be completed and that all hazardous ingredients meeting certain criteria be listed subject to exemptions granted under the Hazardous Materials Information Review Act. The MSDS can be found easily on the internet from different chemical supplier in different formats. After reading the MSDS, we will be aware of the level of risk and required personal protection when we handles these chemicals. The reaction chosen for the isothermal demonstration is the saponification of ethyl acetate by sodium hydroxide, as it can be carried out under safe conditions of temperature and pressure and is well documented. Dilution of Ethyl Acetate use of a 0.1M solution of Ethyl Acetate, therefore add 4.895ml of concentrated Ethyl Acetate to 400ml of deionised or distilled water. Shake the mixture vigorously until the two liquids have mixed. Add further water to make up the final volume to 500ml. Dilution of Sodium Hydroxide use of a 0.1M solution of Sodium Hydroxide, this may be made by adding 50ml of NaOH IM to 450ml of distilled water then making up the solution to 500ml. https://web.microsoftstream.com/video/d836d4ca-d78a-4cc6-9abl-28c443b30619 Preparation of NaOH calibration curve https://web.microsoftstream.cem/video/5fc04323-2965-4c1b-a09d-edf56531c293 Calibration curve is very useful tool in reading the change of the concentration of reactants during the chemical reaction. In order to prepare the calibration curve, we need to prepare different solutions of NaOH ranging from 00.1M, such as ( 0 (pure water), 0.02,0.04,0.06, 0.08 and 0.1 . Read the conductivity for each solution 3 times. Make a table as below. Report 1: After calculating the average conductivity, use excel sheet to plot Average conductivity vs NaOH molarity. Later we will use this curve to convert the conductivity from experiment into concentration. What to include in my report? 1. Report is made by 3 members (maximum 6 pages A4 using MS Word). The report should include cover page, summary, objectives, data collected, results and discussions and conclusion. The report is written using 12pt Times New Romans. Use 1.5 spacing, and 2.5cm margins in all sides. The report is submitted in pdf format. In the first page show the pereent contribution for all members in the team. 2. Write the experiment safety instructions and report from MSDS the safety for handling the chemical reagents in the experimentations. Calibration curve is very useful tool in reading the change of the concentration of reactants during the chemical reaction. In order to prepare the calibration curve, we need to prepare different solutions of NaOH ranging from 00.1M, such as ( 0 (pure water), 0.02,0.04,0.06, 0.08 and 0.1 . Read the conductivity for each solution 3 times. Make a table as below. Report 1: After calculating the average conductivity, use excel sheet to plot Average conductivity vs NaOH molarity. Later we will use this curve to convert the conductivity from experiment into concentration. What to include in my report? 1. Report is made by 3 members (maximum 6 pages A4 using MS Word). The report should include cover page, summary, objectives, data collected, results and discussions and conclusion. The report is written using 12pt Times New Romans. Use 1.5 spacing, and 2.5cm margins in all sides. The report is submitted in pdf format. In the first page show the percent contribution for all members in the team. 2. Write the experiment safety instructions and report from MSDS the safety for handling the chemical reagents in the experimentations. 3. Show the data collected and the correlation between conductivity and molarity (calibration curve) Experiment 1 Chemical Reagent Preparation Objective Prepare the chemical solutions required for the reaction rate testing including a calibration curve for the NaOH reagent. What is MSDS? Before start preparing the chemical reagents that will be used in the experiment, we need to obtain the material safety data sheet (MSDS) for all reagents in the experiment. A material safety data sheet is a technical document which provides detailed and comprehensive information on a controlled product related to - health effects of exposure to the product - hazard evaluation related to the product's handling, storage or use - measure to protect workers at risk of exposure - emergency procedures The data sheet may be written, printed or otherwise expressed and must meet the availability, design and content requirements of Workplace Hazardous Materials Information System (WHMIS) legislation. The legislation provides for flexibility of design and wording but requires that a minimum number of categories of information be completed and that all hazardous ingredients meeting certain criteria be listed subject to exemptions granted under the Hazardous Materials Information Review Act. The MSDS can be found easily on the internet from different chemical supplier in different formats. After reading the MSDS, we will be aware of the level of risk and required personal protection when we handles these chemicals. The reaction chosen for the isothermal demonstration is the saponification of ethyl acetate by sodium hydroxide, as it can be carried out under safe conditions of temperature and pressure and is well documented. Dilution of Ethyl Acetate use of a 0.1M solution of Ethyl Acetate, therefore add 4.895ml of concentrated Ethyl Acetate to 400ml of deionised or distilled water. Shake the mixture vigorously until the two liquids have mixed. Add further water to make up the final volume to 500ml. Dilution of Sodium Hydroxide use of a 0.1M solution of Sodium Hydroxide, this may be made by adding 50ml of NaOH IM to 450ml of distilled water then making up the solution to 500ml. https://web.microsoftstream.com/video/d836d4ca-d78a-4cc6-9abl-28c443b30619 Preparation of NaOH calibration curve https://web.microsoftstream.cem/video/5fc04323-2965-4c1b-a09d-edf56531c293 Calibration curve is very useful tool in reading the change of the concentration of reactants during the chemical reaction. In order to prepare the calibration curve, we need to prepare different solutions of NaOH ranging from 00.1M, such as ( 0 (pure water), 0.02,0.04,0.06, 0.08 and 0.1 . Read the conductivity for each solution 3 times. Make a table as below. Report 1: After calculating the average conductivity, use excel sheet to plot Average conductivity vs NaOH molarity. Later we will use this curve to convert the conductivity from experiment into concentration. What to include in my report? 1. Report is made by 3 members (maximum 6 pages A4 using MS Word). The report should include cover page, summary, objectives, data collected, results and discussions and conclusion. The report is written using 12pt Times New Romans. Use 1.5 spacing, and 2.5cm margins in all sides. The report is submitted in pdf format. In the first page show the pereent contribution for all members in the team. 2. Write the experiment safety instructions and report from MSDS the safety for handling the chemical reagents in the experimentations. Calibration curve is very useful tool in reading the change of the concentration of reactants during the chemical reaction. In order to prepare the calibration curve, we need to prepare different solutions of NaOH ranging from 00.1M, such as ( 0 (pure water), 0.02,0.04,0.06, 0.08 and 0.1 . Read the conductivity for each solution 3 times. Make a table as below. Report 1: After calculating the average conductivity, use excel sheet to plot Average conductivity vs NaOH molarity. Later we will use this curve to convert the conductivity from experiment into concentration. What to include in my report? 1. Report is made by 3 members (maximum 6 pages A4 using MS Word). The report should include cover page, summary, objectives, data collected, results and discussions and conclusion. The report is written using 12pt Times New Romans. Use 1.5 spacing, and 2.5cm margins in all sides. The report is submitted in pdf format. In the first page show the percent contribution for all members in the team. 2. Write the experiment safety instructions and report from MSDS the safety for handling the chemical reagents in the experimentations. 3. Show the data collected and the correlation between conductivity and molarity (calibration curve) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


