Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve this question using gauss newton method The following table shows the reaction rate and temperature values for nitrogen dioxide, or NO2: T(K) 592 604
solve this question using gauss newton method 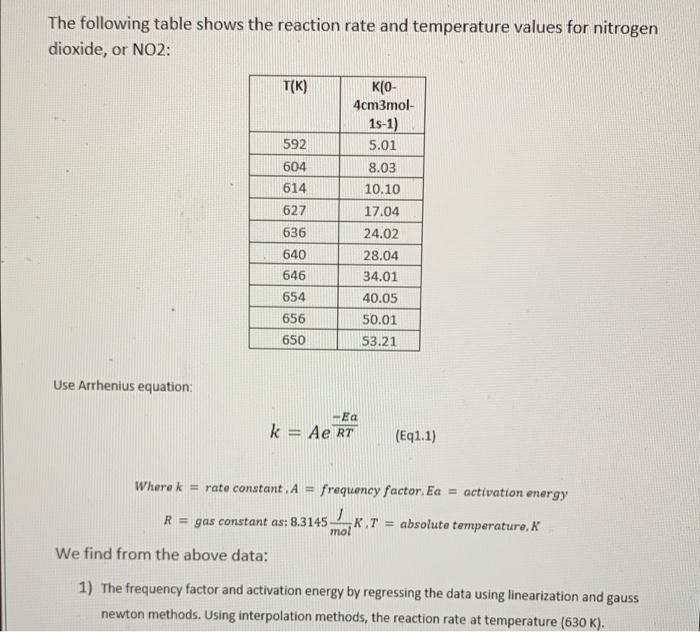
The following table shows the reaction rate and temperature values for nitrogen dioxide, or NO2: T(K) 592 604 614 627 636 640 646 654 656 KO- 4cm3mol- 1s-1) 5.01 8.03 10.10 17.04 24.02 28.04 34.01 40.05 50.01 53.21 650 Use Arrhenius equation: k = Ae RT (Eq1.1) Where k = rate constant. A frequency factor. Ea = activation energy R = gas constant as: 8.3145 LKT = absolute temperature, mol We find from the above data: 1) The frequency factor and activation energy by regressing the data using linearization and gauss newton methods. Using interpolation methods, the reaction rate at temperature (630 K) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


