Question
Solve using MATLAB You should get same answers as worked out T=313 K 1) Use the Van der Waals EOS and calculate the PV value
Solve using MATLAB
You should get same answers as worked out
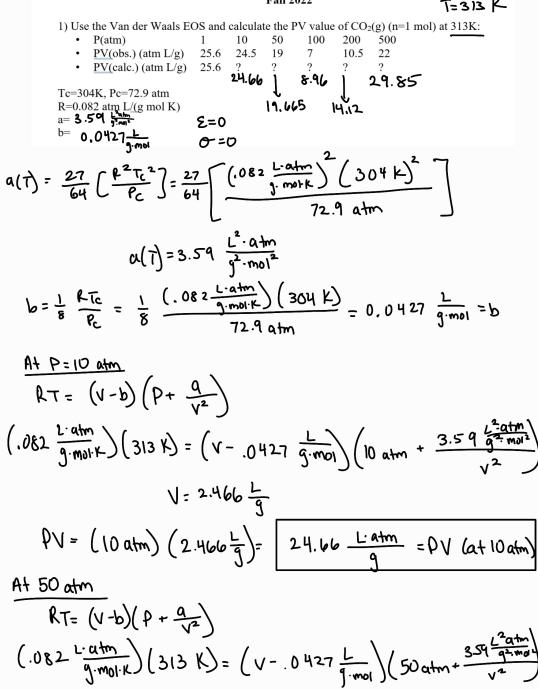

T=313 K 1) Use the Van der Waals EOS and calculate the PV value of CO2(g) (n-1 mol) at 313K: = b= 0.0427 mol P(atm) PV(obs.) (atm L/g) 1 10 50 100 200 500 25.6 24.5 19 7 10.5 22 PV(calc.) (atm L/g) 25.6 ? ? ? ? ? 24.66 Te-304K, Pc-72.9 atm 18:96 29.85 R-0.082 atm L/(g mol K) 19.665 14.12 a= 3.59 E=0 0=0 2 (.082 Latin J. mork 72.9 atm m (304k) (30+K)] L. atm g-mol a(7)- 27 (127 = 27 Pc 9(7)=3.59 b= RTC = (082 LE) (304 k). Pc A+ P=10 atm 8 RT= (v-b) (P + 1) 72.9 atm = 0.0427 =b g.mol (.082 Lambork ) ( 313 k) = (v- 0427 ima) ix) (313 K) (v-.0427 Latm gmor v V= 2.466 6615 15 m) (0 lm + 3.59 (10 10 atm PV = (10 atm) (2.466 5 ) = 24.66 Latm = PV (a+10 atm) At 50 atm RT= (v-b) (P + =) L-atm (.082 ) (313 k) = (v-.0427 mm) (50 atm- gmolk. mol 3.59 atm gamoy
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus Early Transcendentals
Authors: William L. Briggs, Lyle Cochran, Bernard Gillett
2nd edition
321954428, 321954424, 978-0321947345
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



