Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solved where? if you are wasting my time, i'll report you to chegg customer service, thanks! Process Analysis Problem 1: (60 MARKS] Background: Hydrogen is
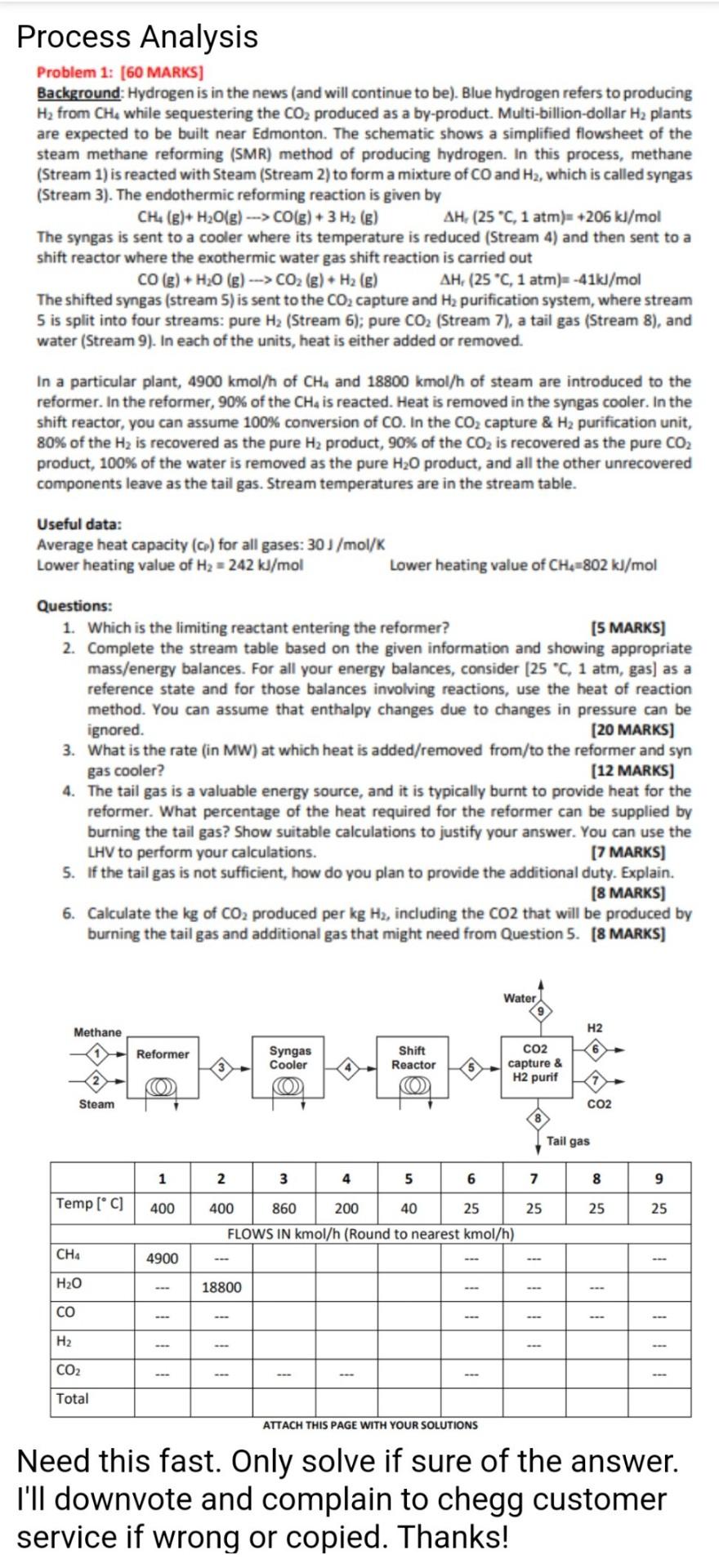
solved where? if you are wasting my time, i'll report you to chegg customer service, thanks!
Process Analysis Problem 1: (60 MARKS] Background: Hydrogen is in the news (and will continue to be). Blue hydrogen refers to producing H2 from CHA while sequestering the CO2 produced as a by-product. Multi-billion-dollar H2 plants are expected to be built near Edmonton. The schematic shows a simplified flowsheet of the steam methane reforming (SMR) method of producing hydrogen. In this process, methane (Stream 1) is reacted with Steam (Stream 2) to form a mixture of CO and H2, which is called syngas (Stream 3). The endothermic reforming reaction is given by CH, (E)+ H2O(g) ---> CO(g) + 3 H2 (E) AH, (25 C, 1 atm) +206 kJ/mol The syngas is sent to a cooler where its temperature is reduced (Stream 4) and then sent to a shift reactor where the exothermic water gas shift reaction is carried out CO(g) + H20 (6) ---> CO2 (g) + H2 (6) AH(25 C, 1 atm) -41kJ/mol The shifted syngas (stream 5) is sent to the CO2 capture and Hz purification system, where stream 5 is split into four streams: pure H2 (Stream 6); pure CO2 (Stream 7), a tail gas (Stream 8), and water (Stream 9). In each of the units, heat is either added or removed. In a particular plant, 4900 kmol/h of CH4 and 18800 kmol/h of steam are introduced to the reformer. In the reformer, 90% of the CH4 is reacted. Heat is removed in the syngas cooler. In the shift reactor, you can assume 100% conversion of CO. In the CO2 capture & H2 purification unit, 80% of the Hz is recovered as the pure H2 product, 90% of the CO2 is recovered as the pure CO2 product, 100% of the water is removed as the pure H20 product, and all the other unrecovered components leave as the tail gas. Stream temperatures are in the stream table. Useful data: Average heat capacity (o) for all gases: 30J/mol/K Lower heating value of H2 = 242 kJ/mol Lower heating value of CH4=802 kJ/mol Questions: 1. Which is the limiting reactant entering the reformer? (5 MARKS] 2. Complete the stream table based on the given information and showing appropriate mass/energy balances. For all your energy balances, consider (25 C, 1 atm, gas) as a reference state and for those balances involving reactions, use the heat of reaction method. You can assume that enthalpy changes due to changes in pressure can be ignored. [20 MARKS] 3. What is the rate (in MW) at which heat is added/removed from/to the reformer and syn gas cooler? (12 MARKS] 4. The tail gas is a valuable energy source, and it is typically burnt to provide heat for the reformer. What percentage of the heat required for the reformer can be supplied by burning the tail gas? Show suitable calculations to justify your answer. You can use the LHV to perform your calculations. [7 MARKS] 5. If the tail gas is not sufficient, how do you plan to provide the additional duty. Explain. [8 MARKS] 6. Calculate the kg of CO2 produced per kg Hz, including the CO2 that will be produced by burning the tail gas and additional gas that might need from Question 5. (8 MARKS] Water Methane H2 Reformer Syngas Cooler O Reactor CO2 capture & H2 purif O Steam CO2 Tail gas 1 2 6 7 8 9 Temp [C] 400 25 25 25 400 860 200 40 25 FLOWS IN kmol/h (Round to nearest kmol/h) CHA 4900 H2O 18800 CO H2 CO2 Total ATTACH THIS PAGE WITH YOUR SOLUTIONS Need this fast. Only solve if sure of the answer. I'll downvote and complain to chegg customer service if wrong or copied. ThanksStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


