Answered step by step
Verified Expert Solution
Question
1 Approved Answer
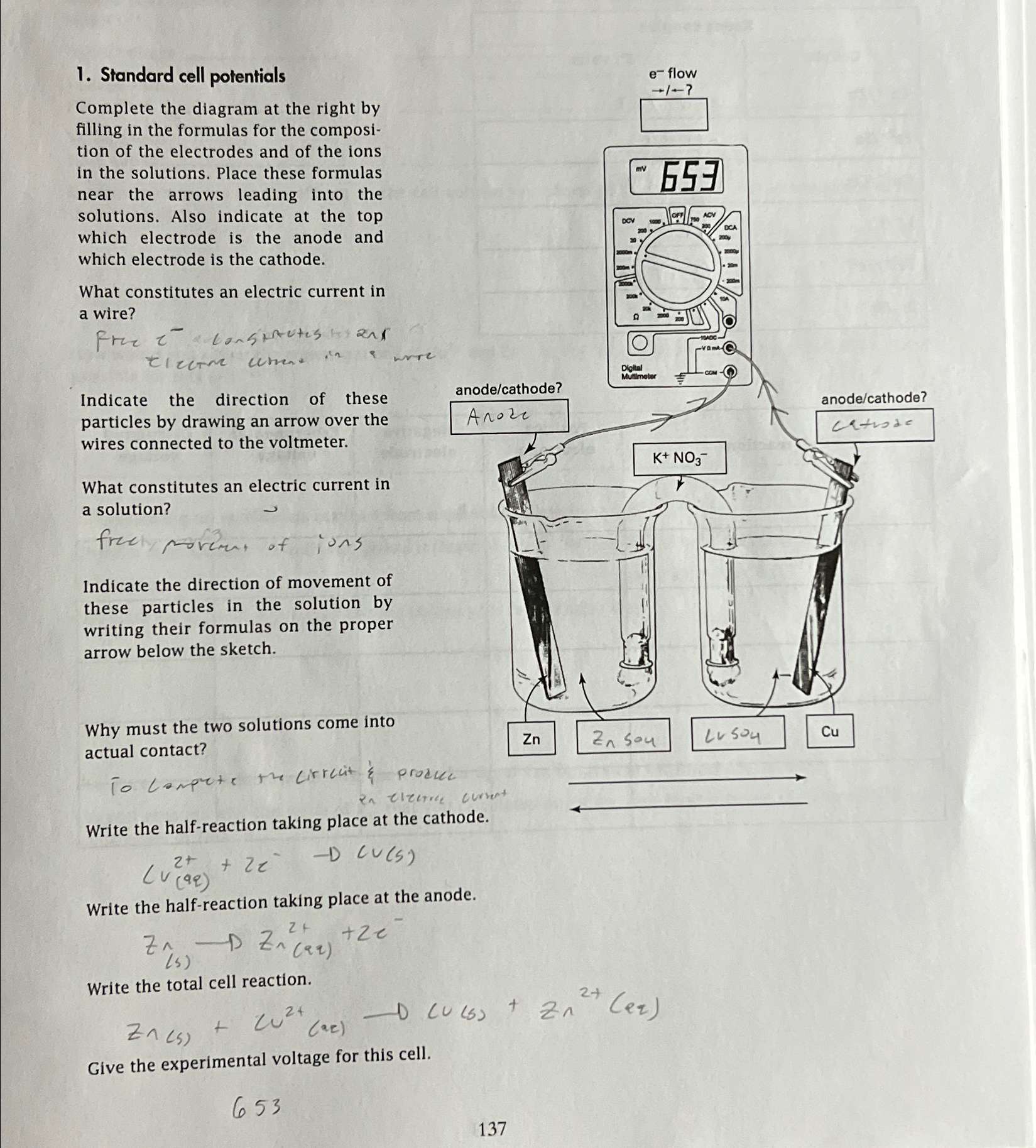
Standard cell potentials Complete the diagram at the right by filling in the formulas for the composition of the electrodes and of the ions in
Standard cell potentials
Complete the diagram at the right by filling in the formulas for the composition of the electrodes and of the ions in the solutions. Place these formulas near the arrows leading into the solutions. Also indicate at the top which electrode is the anode and which electrode is the cathode.
What constitutes an electric current in a wire?
Free conspaters and
Electre current in a wrre
Indicate the direction of these particles by drawing an arrow over the wires connected to the voltmeter.
What constitutes an electric current in a solution?
freel moreut of ions
Indicate the direction of movement of these particles in the solution by writing their formulas on the proper arrow below the sketch.
Why must the two solutions come into actual contact?
Campete the Circuit
Write the halfreaction taking place at the anode.
Write the halfreaction taking place at the cathode.
Write the total cell reaction.
Give the experimental voltage for this cell.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


