Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Starting with 2-methylpropene (isobutylene) list in order (by letter, no period) the reagents required for the synthesis of each of the following (T= tritium,
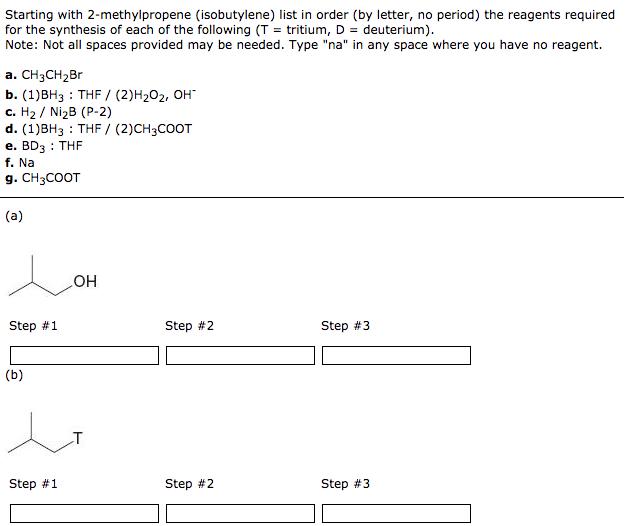
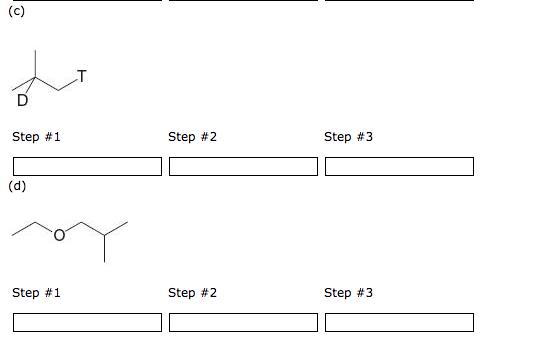

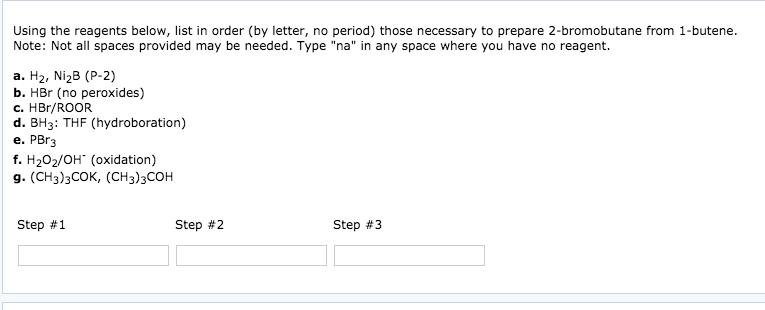
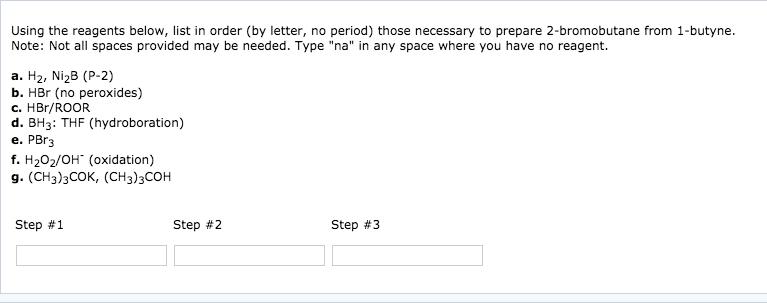
Starting with 2-methylpropene (isobutylene) list in order (by letter, no period) the reagents required for the synthesis of each of the following (T= tritium, D = deuterium). Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. CH3CHBr b. (1)BH3: THF / (2) HO, OH c. H / NiB (P-2) d. (1)BH3: THF / (2)CH3COOT e. BD3: THF f. Na g. CH3COOT (a) Step #1 (b) Step #1 OH Step #2 Step #2 Step #3 Step #3 (c) Step #1 (d) Step #1 Step #2 Step #2 Step #3 Step #3 Draw the structure of the products that would be formed when cyclopentanol reacts with p-toluenesulfonyl chloride, then base followed by KI. Product containing iodine: ? Edit Product not containing iodine: ? Edit Using the reagents below, list in order (by letter, no period) those necessary to prepare 2-bromobutane from 1-butene. Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. H, NiB (P-2) b. HBr (no peroxides) c. HBr/ROOR d. BH3: THF (hydroboration) e. PBr3 f. HO/OH (oxidation) g. (CH3)3COK, (CH3)3COH Step #1 Step #2 Step #3 Using the reagents below, list in order (by letter, no period) those necessary to prepare 2-bromobutane from 1-butyne. Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. H, NiB (P-2) b. HBr (no peroxides) c. HBr/ROOR d. BH3: THF (hydroboration) e. PBr3 f. HO/OH (oxidation) g. (CH3)3COK, (CH3)3COH Step #1 Step #2 Step #3 Starting with 2-methylpropene (isobutylene) list in order (by letter, no period) the reagents required for the synthesis of each of the following (T= tritium, D = deuterium). Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. CH3CHBr b. (1)BH3: THF / (2) HO, OH c. H / NiB (P-2) d. (1)BH3: THF / (2)CH3COOT e. BD3: THF f. Na g. CH3COOT (a) Step #1 (b) Step #1 OH Step #2 Step #2 Step #3 Step #3 (c) Step #1 (d) Step #1 Step #2 Step #2 Step #3 Step #3 Draw the structure of the products that would be formed when cyclopentanol reacts with p-toluenesulfonyl chloride, then base followed by KI. Product containing iodine: ? Edit Product not containing iodine: ? Edit Using the reagents below, list in order (by letter, no period) those necessary to prepare 2-bromobutane from 1-butene. Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. H, NiB (P-2) b. HBr (no peroxides) c. HBr/ROOR d. BH3: THF (hydroboration) e. PBr3 f. HO/OH (oxidation) g. (CH3)3COK, (CH3)3COH Step #1 Step #2 Step #3 Using the reagents below, list in order (by letter, no period) those necessary to prepare 2-bromobutane from 1-butyne. Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. H, NiB (P-2) b. HBr (no peroxides) c. HBr/ROOR d. BH3: THF (hydroboration) e. PBr3 f. HO/OH (oxidation) g. (CH3)3COK, (CH3)3COH Step #1 Step #2 Step #3
Step by Step Solution
★★★★★
3.36 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
1 Answers are filled in the blan...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


