Answered step by step
Verified Expert Solution
Question
1 Approved Answer
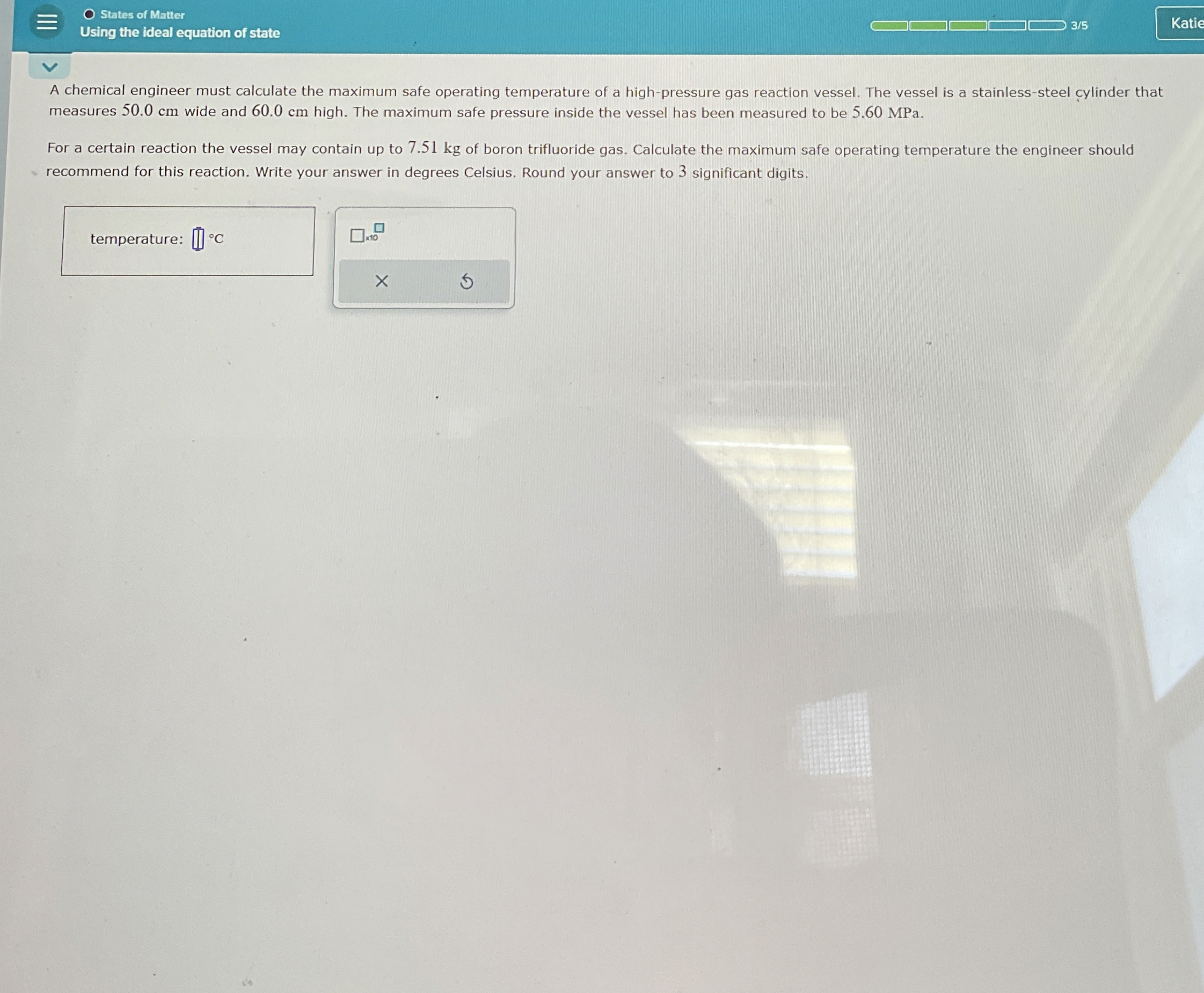
States of Matter Using the ideal equation of state A chemical engineer must calculate the maximum safe operating temperature of a high - pressure gas
States of Matter
Using the ideal equation of state
A chemical engineer must calculate the maximum safe operating temperature of a highpressure gas reaction vessel. The vessel is a stainlesssteel cylinder that measures wide and high. The maximum safe pressure inside the vessel has been measured to be MPa.
For a certain reaction the vessel may contain up to of boron trifluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to significant digits.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


