Answered step by step
Verified Expert Solution
Question
1 Approved Answer
stban Tom 1 p (kPa) p (kPa) 0.565 boom 0.893 isosteric heat of adsorption. 12.5 (Langmuir isotherm for two adsorbates in a mixture) Nakahara et
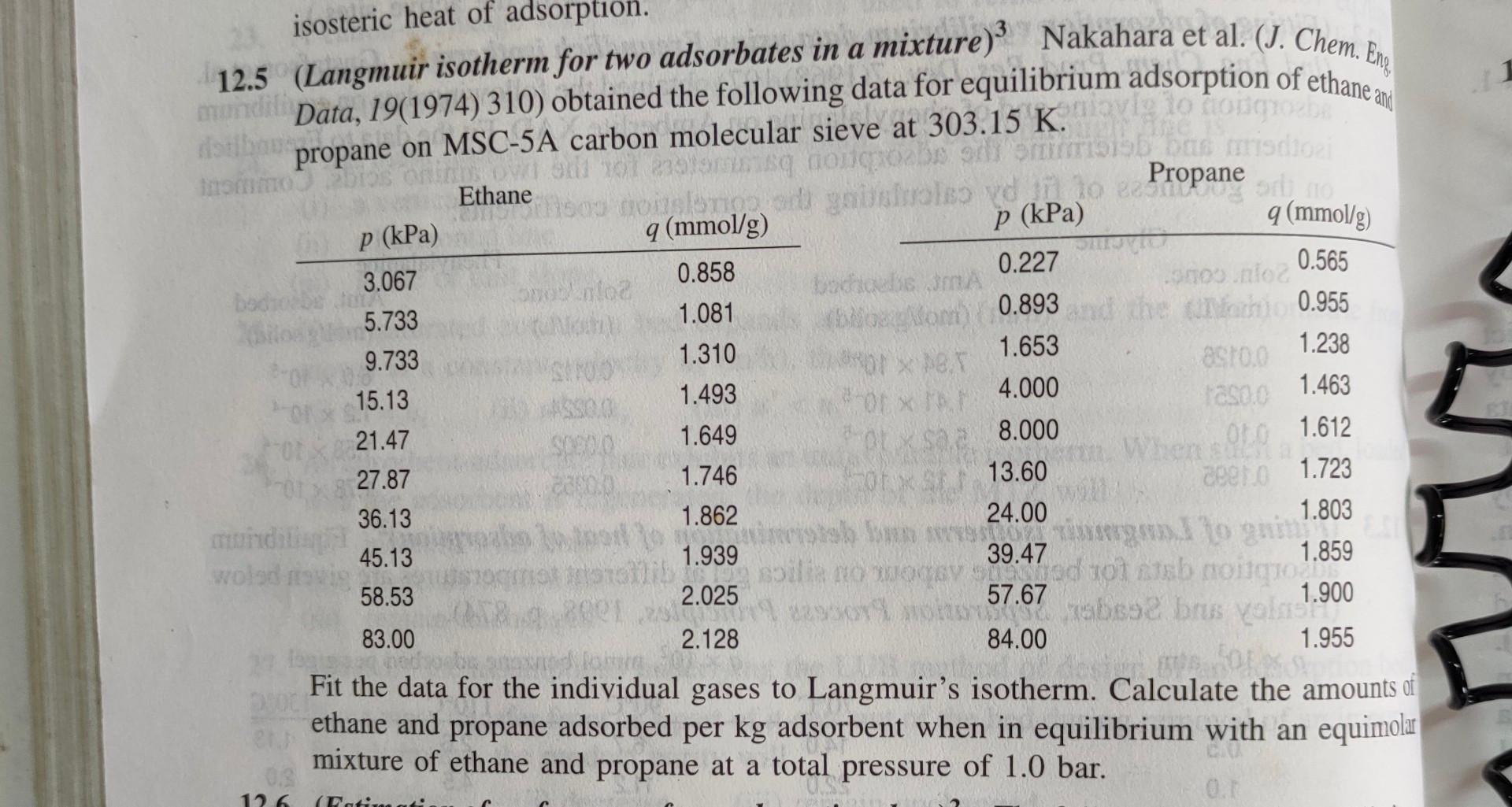
stban Tom 1 p (kPa) p (kPa) 0.565 boom 0.893 isosteric heat of adsorption. 12.5 (Langmuir isotherm for two adsorbates in a mixture) Nakahara et al. (J. Chem. En mundi Data, 19(1974)310) obtained the following data for equilibrium adsorption of ethane an V2 to GOLD propane on MSC-5A carbon molecular sieve at 303.15 K. o or doua obe / ommibus mariscopi Ethane voiylar gnisolo vdano e Fropane or to q (mmol/g) 9 (mmol/g) 0.227 3.067 0.858 Coloa bodo bodas -9500 loc 5.733 1.081 che Machi 0.955 9.733 1.310 1.653 1.238 asro 15.13 1.493 * 4.000 Tasa. 1.463 21.47 1.649 for saa 8.000 1.612 27.87 1.746 13.60 0 36.13 1.862 24.00 1.803 mandil Sebas igen. I to give in 45.13 1.939 39.47 wold bolla 1.859 od rottsb motion 58.53 2.025 57.67 1.900 absa brus Valka 83.00 2.128 84.00 1.955 BE TUS 300 Fit the data for the individual gases to Langmuir's isotherm. Calculate the amounts of ethane and propane adsorbed per kg adsorbent when in equilibrium with an equimolar mixture of ethane and propane at a total pressure of 1.0 bar. CA ceto 1.723 126 (Patina
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


