Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Steel is being made in an L.D. converter. The metallic charge consists of hot metal and scrap in the ratio of 4: 1. The
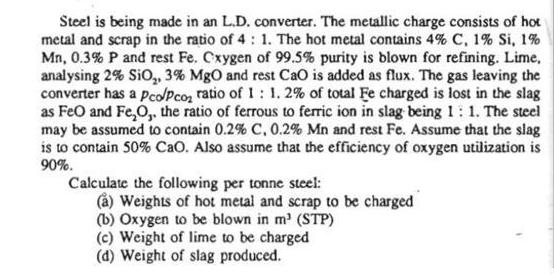
Steel is being made in an L.D. converter. The metallic charge consists of hot metal and scrap in the ratio of 4: 1. The hot metal contains 4% C, 1% Si, 1% Mn, 0.3% P and rest Fe. Cxygen of 99.5% purity is blown for refining. Lime, analysing 2% SiO, 3% MgO and rest CaO is added as flux. The gas leaving the converter has a pco/Pco ratio of 1: 1. 2% of total Fe charged is lost in the slag as FeO and FeO,, the ratio of ferrous to ferric ion in slag being 1: 1. The steel may be assumed to contain 0.2% C, 0.2% Mn and rest Fe. Assume that the slag is to contain 50% CaO. Also assume that the efficiency of oxygen utilization is 90%. Calculate the following per tonne steel: (a) Weights of hot metal and scrap to be charged (b) Oxygen to be blown in m (STP) (c) Weight of lime to be charged (d) Weight of slag produced.
Step by Step Solution
★★★★★
3.51 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


