Question: Strongest Bonding A B C Weakest Covalent (A)-> Metallic (B) -> Ionic (C) -> Van Der Waals (D) Ionic (A) -> Covalent (B) ->
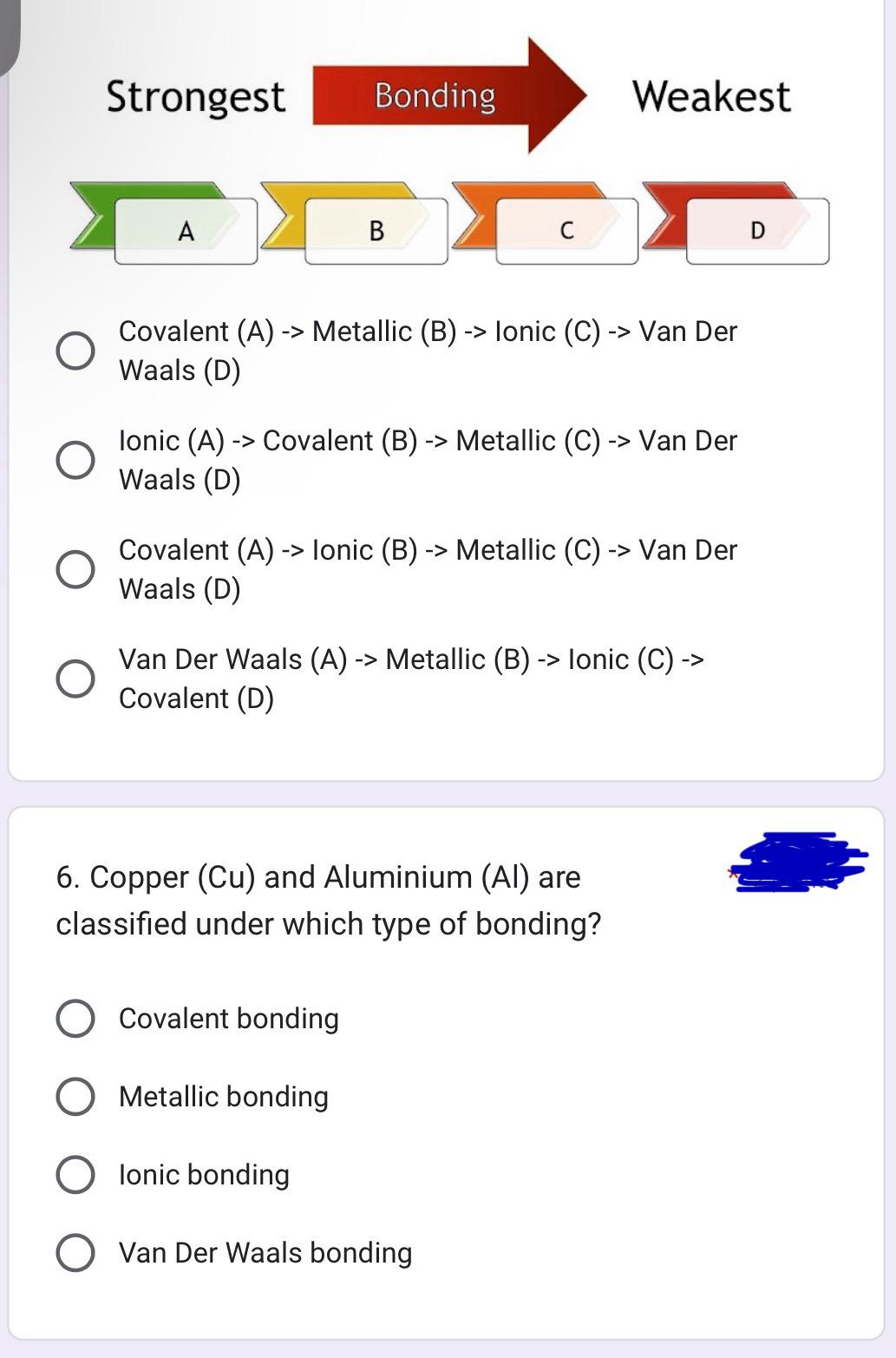
Strongest Bonding A B C Weakest Covalent (A)-> Metallic (B) -> Ionic (C) -> Van Der Waals (D) Ionic (A) -> Covalent (B) -> Metallic (C) -> Van Der Waals (D) Covalent (A) -> Ionic (B) -> Metallic (C) -> Van Der Waals (D) Van Der Waals (A) -> Metallic (B) -> Ionic (C) -> Covalent (D) 6. Copper (Cu) and Aluminium (Al) are classified under which type of bonding? Covalent bonding Metallic bonding Ionic bonding Van Der Waals bonding
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


