Answered step by step
Verified Expert Solution
Question
1 Approved Answer
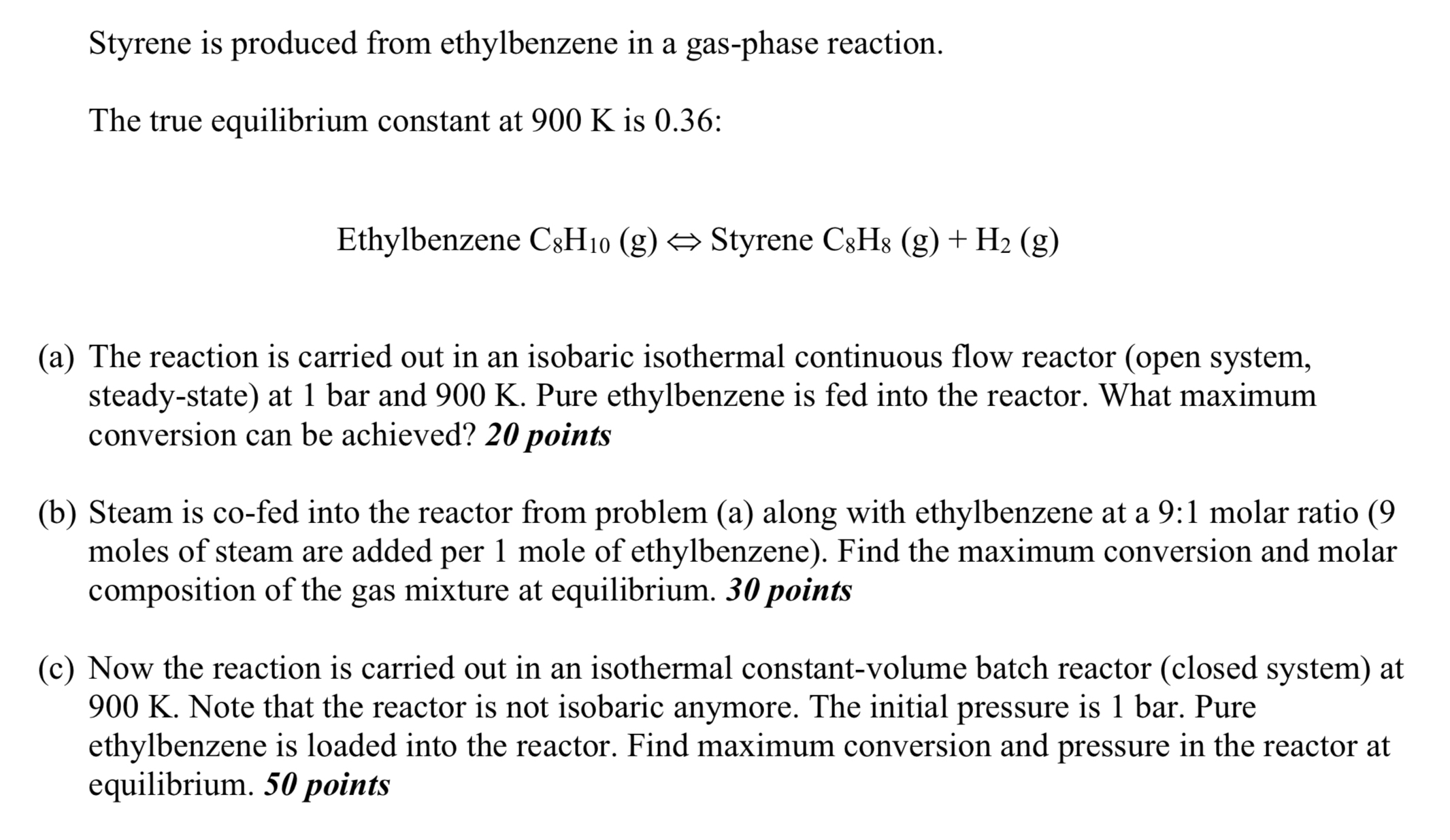
Styrene is produced from ethylbenzene in a gas - phase reaction. The true equilibrium constant at 9 0 0 K is 0 . 3 6
Styrene is produced from ethylbenzene in a gasphase reaction.
The true equilibrium constant at is :
Ethylbenzene Styrene
a The reaction is carried out in an isobaric isothermal continuous flow reactor open system, steadystate at bar and Pure ethylbenzene is fed into the reactor. What maximum conversion can be achieved? points
b Steam is cofed into the reactor from problem a along with ethylbenzene at a : molar ratio moles of steam are added per mole of ethylbenzene Find the maximum conversion and molar composition of the gas mixture at equilibrium. points
c Now the reaction is carried out in an isothermal constantvolume batch reactor closed system at Note that the reactor is not isobaric anymore. The initial pressure is Pure ethylbenzene is loaded into the reactor. Find maximum conversion and pressure in the reactor at equilibrium. points
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


