Question
3.1 A piston-cylinder device contains a liquid-vapor mixture of water at 300 K. During a constant-pressure process, 750 kJ of heat is transferred to
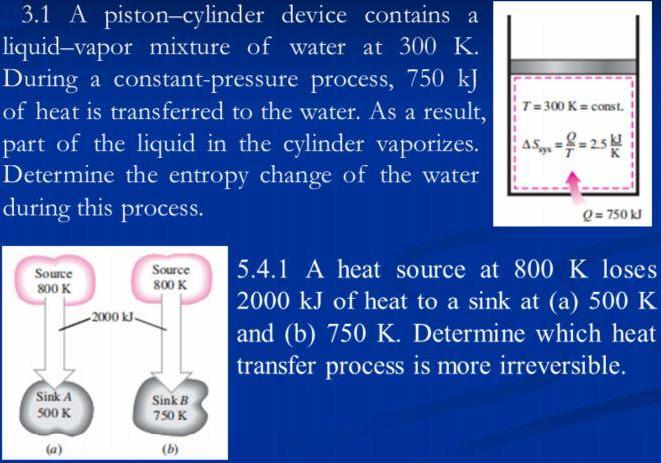
3.1 A piston-cylinder device contains a liquid-vapor mixture of water at 300 K. During a constant-pressure process, 750 kJ of heat is transferred to the water. As a result, T= 300 K= const. part of the liquid in the cylinder vaporizes. Determine the entropy change of the water during this process. AS==25 Q= 750 kJ Source Source 5.4.1 A heat source at 800 K loses 800 K 800 K 2000 kJ of heat to a sink at (a) 500 K and (b) 750 K. Determine which heat -2000 kl- transfer process is more irreversible. Sink A Sink B 750 K 500 K (a) (b)
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



